Organoarsenic compounds have been known for over 200 years and in that time have given insight into important theoretical topics in chemistry - from valency to aromaticity. They have also proved to have beneficial pharmacological effects.
-
The syntheses of organoarsenic compounds led Edward Frankland to the concept of valency, and Paul Ehrlich to a treatment for syphilis
Arsenic is a member of the nitrogen group of the Periodic Table, forming compounds in both the +III and +V oxidation states. Unlike some metals, arsenic also forms organometallic compounds in both oxidation states, R3 As and R5 As.1,2 The R3 As compounds are pseudo tetrahedral (like ammonia), and where three different organic groups are present, optical isomers of the type R1 R2 R3 As are known. Although generally synthetic, there are naturally occurring organoarsenic compounds. For example the arsenobetaine Me3 As+ CH2 CO2 has been found in the tail of the western rock lobster3 and in the muscle and liver of the shark Prionace glaucus.4
Early history and the concept of valency
The first organometallic compound to be identified was tetramethyl-diarsine (1).
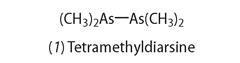
This poisonous oily liquid was obtained by French chemist, Louis Claude Cadet in 1760, by heating arsenic oxide with potassium acetate (ethanoate) and then distilling out the product. Called 'kakodyl' (later cacodyl), from the Greek meaning 'evil-smelling liquid', its composition was established by Robert Wilhelm Bunsen in 1843.

Bunsen began to study the chemistry of cacodyl in the late 1830s when he was at the University of Marburg. In the days before fume cupboards, this was a dangerous undertaking. Cacodyl is poisonous, flammable and has a nauseating odour, even at low concentrations. Bunsen wrote of cacodyl: 'the smell of this body produces instantaneous tingling of the hands and feet and even giddiness and insensibility'. He went on to observe that 'when one is exposed to the smell. the tongue becomes covered with a black coating'.
Bunsen worked quickly and, to overcome the smell of cacodyl, did his experiments while breathing through a long glass tube that extended to the outside of his laboratory. Despite these precautions, he nearly killed himself from arsenic poisoning, and lost the sight of one eye in a painful accident when a sample of cacodyl exploded and shattered a glass vessel close to his face.5

While at Marburg, Bunsen was joined briefly by the young English chemist Edward Frankland.6 In 1849 Frankland, by using gas handling techniques he had learnt from Bunsen, thought he had isolated ethyl radicals. Indeed, for a short while he was introduced to Marburg society as the 'discoverer of ethyl'. In fact, what he had done was to prepare butane by the action of water on diethylzinc.
Working alongside Bunsen, Frankland focused his efforts on synthesising organometallic compounds. In 1854 he published his results in the Journal of the Chemical Society.7 Experimental details are sketchy, but appear to have involved heating the finely divided element - Main Group metals such as zinc, tin, arsenic, antimony and mercury - with excess of organic iodide. He sealed the reactants in a glass tube and heated them on an oil bath, or with light. In the latter case, he concentrated sunlight by using a parabolic reflector, and placed the sealed glass tube containing the reactants at its focus. Frankland had no way of knowing the correct equivalent weight of carbon, so following the convention of the time, he took it to be six, and thus wrote methyl as C2H3. While it is possible to identify the compounds he synthesised it is less clear for arsenic, but he seems to have prepared the tetra-alkyldiarsines for R = ethyl, propyl, butyl and pentyl. He also made tetraphenyldiarsine. He thus extended the range of known organoarsenic compounds beyond cacodyl (R = methyl).
This work was significant for two reasons. First, it was the earliest explicit recognition of the existence of organometallic compounds and, indeed, Frankland himself coined the word 'organometallic' to describe them.7 Secondly, by noticing the number of organic groups that could be attached to individual metal atoms, Frankland was led towards the concept of valency.6,8 Towards the end of his 1854 paper, for instance, he notes a degree of similarity between formulae then used for compounds such as NO3, NH3, PO3, SbCl3 and AsH3, suggesting that 'the combining power of the attracting element, if I may be allowed the term, is always satisfied by the same number of these atoms'.7 He was criticised by his peers because not all the formulae were correct but, to be fair, at the time there were many unresolved issues concerning the relative atomic mass. However, the essential concept that elements have specific 'combining power' was born and, without question, the concept first saw the light of day in Frankland's mind in the early 1850s.
Salvarsan and Ehrlich's 'magic bullet'
Paul Ehrlich (1854-1915) was a distinguished German bacteriologist who, early in his life, became fascinated with the fact that certain dyes were selectively taken up by blood cells and the cells of other tissues. He was particularly interested by the action of such compounds on parasites that caused disease, and in 1891 he reported the successful use of methylene blue as a novel treatment for malaria.

Ehrlich continued to search for dyes which acted as bacterial toxins, which he called 'magic bullets' - ie individual compounds that would target and destroy the microorganisms responsible for diseases. He had little success with other dyes so he turned his attention to other compounds. Spurred on by earlier work by Robert Koch, who pioneered tuberculin therapy for tuberculosis and Emil von Behring who developed therapies based on sera for the treatment of tetanus and diptheria, Ehrlich turned his attention to a treatment for syphilis.
The microorganism responsible for syphilis is the bacterium Treponema pallidum. Ehrlich brought together a team of scientists, including the chemist Alfred Bertheim and the microbiologist Sahachiro Hata, with the aim of developing a magic bullet that would kill this bacterium without affecting the human host. They chose an organoarsenic compound as their starting point, and Bertheim synthesised several hundred related compounds. Number 606 - arsphenamine (dioxydiamido-arsenobenzenol) - proved the most effective and a single dose completely cured rabbits infected with the bacterium. The compound was then tested on patients who had the disease at an advanced stage, when the dementia associated with its final phase had set in. To their surprise, several of these 'terminal' patients made a full recovery. Compound 606, prepared and tested in the autumn of 1909, was in clinical use under the name salvarsan by 1910. Salvarsan remained the treatment of choice for syphilis until the development of antibiotics in the 1940s. Ehrlich received the Nobel prize for medicine for this work in 1907.9 (The story of his triumph was the subject of a film, Dr Ehrlich's magic bullet, made in 1940 and starring Edward G. Robinson.)
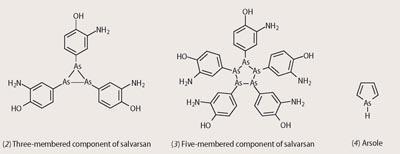
Despite its clinical success, uncertainty remained over the structure of salvarsan. The original synthesis involved the reduction of 3-nitro-4-hydroxyphenylarsonic acid with dithionite, yielding a compound that could be written as RAs.HCl.H2O (R = 3-amino-4 hydroxyphenyl). By analogy with azo compounds, Ehrlich and his team suggested that salvarsan contained an As=As double bond. In 2005 this was challenged. Bryan Nicholson and his team at the University of Waikato in New Zealand showed that salvarsan is a mixture of three- and five-membered ring structures based on As-As bonded species (see structures (2) and (3)).10 This mixture appears to undergo slow hydrolysis to yield RAs(OH)2, and this, according to Nicholson, is the compound that is active against T. pallidum. Research continues to determine exactly what makes this compound quite so specific against this bacterium.
Aromaticity
That arsenic is a member of the nitrogen group of the Periodic Table led several chemists to consider preparing arsenic analogues of nitrogen heterocycles. Of note were Eustace E. Turner (1893-1966) and George J. Burrows (1888-1950) at the University of Sydney in the 1920s.
After graduating in 1913 from East London College (now Queen Mary College, University of London), and before he took his principle appointment as professor of chemistry at Bedford College London,11 Turner spent two years, from 1919 to 1921 at the University of Sydney. Here he made a brief but significant foray into arsenic chemistry. Turner and Burrows prepared the arsenic analogue of indole, a molecule they wanted to call 'arsole'. However, when they submitted their paper to the Journal and Proceedings of the Royal Society of New South Wales, the editor rejected the name.12 A major triumph of the partnership was that they demonstrated optical activity in an arsonium salt for the first time, despite the very fast rate of racemisation that the compound exhibits.
Such was Turner's influence that he effectively established the Sydney school of chemistry as a result of his research into the organic chemistry of arsenic. At that time the University of Sydney did not offer PhDs. However, Turner made it possible for students to transfer to University College London to study for a doctorate. Ronald Nyholm, whose seminal contribution was also to coordination chemistry, was one of the beneficiaries of this scheme. Nyholm also inherited an enthusiasm for organoarsenic chemistry, and pioneered the use of various organic diarsines to stabilise transition metal complexes of unusual oxidation states.
George Burrows continued to do research into organometallic compounds, particularly the coordination of organoarsenic compounds, long after Turner had returned to London. In fact, such was Burrows' distinction in this field that the premier award of the Inorganic Division of the Royal Australian Chemical Institute is named after him.
Turner and Burrows' name 'arsole' is now applied to the arsenic analogue of pyrrole(4).13 The molecule itself has not be synthesised, but various substituted analogues are known. Pyrrole is aromatic, and the question of the possible aromaticity of the arsenic analogue has been studied recently. In 2005 Johansson and Juselius at the University of Helsinki carried out a quantum-mechanical calculation of the structure of this compound, and showed that it is only slightly aromatic.14 The molecule has a distinct bond angle at the arsenic atom, so unlike aromatic molecules, is non-planar. Consequently the arsenic atom is unable to feed its lone pair of electrons completely into the ring. It is thus the geometry of arsole that inhibits the development of aromaticity, making it notably less aromatic than either benzene or pyrrole.
Whatever their future, organoarsenic compounds will remain challenging to work with, because handling them is always going to be potentially hazardous. They must be treated with respect and proper safety procedures are essential.
John Nicholson is professor of biomaterials chemistry in the department of chemical and pharmaceutical sciences at the University of Greenwich, Medway Campus,Chatham, Kent ME4 4TB.
References
- J. L. Wardell in Comprehensive organometallic chemistry, G. Wilkinson, F. G. A. Stone and E. W. Abel (eds), Chap 18, Vol 2. Oxford: Pergamon, 1982.
- A. W. Parkins and R. C. Poller, An introduction to organometallic chemistry. Basingstoke & London: Macmillan, 1986.
- J. S. Edmunds et al, Tetrahedron Lett., 1977, 1543.
- S. Kurosawa et al, Agric. BioI. Chem., 1980, 4, 1993.
- S. G. Schacher, Dictionary of scientific biography, C. C. Gillespie (ed), Vol 2, p 586. New York: Charles Scribner,1970.
- C. A. Russell, Edward Frankland: chemistry, controversy and conspiracy in Victorian England. Cambridge: CUP, 1996.
- E. Frankland, J. Chem. Soc., 1854, 6, 360.
- C. A. Russell, A history of valency. Leicester: University Press, 1971.
- C. E. Dolman, Dictionary of scientific biography, C. C. Gillispie (ed), Vol 4, p 295. New York: Charles Scribner, 1971.
- N. C. Lloyd et al, Angew. Chem. Int. Edn, 2005, 44, 941.
- A. T. Baker and S. T. Livingstone, Polyhedron, 1985, 4, 1337.
- A. Maccoll, Ambix, 1989, 36, 82.
- G. Markl and H. Hauptmann, J. Organomet. Chem., 1983, 248, 269.
- M. P. Johansson and J. Juselius, Lett. Org. Chem., 2005, 2, 469.






No comments yet