This year marks the 100th anniversary of the birth of Leo Sternbach, discoverer of the anti-anxiety drugs Librium and Valium
-
The discovery of the benzodiazepines by Leo Sternbach as anti-anxiety drugs was a 'hit and miss' affair underpinned by a touch of serendipity in the laboratory

This year marks the 100th anniversary of the birth of Leo Sternbach, an organic chemist by training, who is credited with the discovery of the anti-anxiety drugs Librium and Valium. Valium was the most prescribed medicine in the US between 1969 and 1982, and still has an important, but more limited, place in modern treatments.
Anxiety is a common emotion. Indeed, a bit of anxiety helps our concentration and motivation, so that we arrive at work on time, do better in competitions or examinations etc. But too much can be disabling, leaving us unable to make decisions or even tackle daily tasks. Such anxiety may be present continuously, or present intermittently as a panic attack. Sometimes the focus of the anxiety can be a particular thing or situation that others generally do not consider to be a problem, such as spiders or heights, or of being in a crowded place (agoraphobia). Anxiety can leave people leading restricted lives to avoid situations that worsen their symptoms. Such debilitating emotions can affect anyone, including David Beckham and Kim Basinger, who have spoken openly of their struggles with anxiety.[1]
Early promise
The advent of the synthetic drug chlorpromazine[2] in 1952 provided the first effective treatment for schizophrenia, and led pharmaceutical companies to search for new drugs for other psychiatric illnesses. Roche Pharmaceuticals, with laboratories in Nutley, New Jersey, and Basle (Switzerland), directed its research into chemicals that would alleviate chronic anxiety. Until this time the only anti-anxiety medicines available were hypnotics such as chloral hydrate or one of the barbiturates. These helped up to a point, but caused such drowsiness that patients struggled to function properly. And because the therapeutic dose was close to the lethal dose, there was always the risk of a deadly overdose, either by accident or design.

On Roche's staff was Leo Sternbach (see Box).[3] He had studied chemistry at the University of Krakow, and had done post-doctoral research with the professor of chemistry, Karol Dziewonski. From 1937 he worked with Nobel prizewinner Leopold Ruzicka at Zurich's Federal Institute of Technology, in Switzerland. This was a fortuitous move, because Sternbach was a Polish Jew and probably would not have survived the Holocaust when the Nazis over-ran his country in 1939. As it was, Dziewonski and the rest of the university's professorial staff were sent to Sachenshausen concentration camp where they were to spend most of the war. Perhaps this contributed to Sternbach's unease even in neutral Switzerland, for in 1941 (by now working for Roche) he decided to emigrate to the US.
During this time drug research was a 'hit and miss', affair. Companies maintained vast 'libraries' of chemicals, some of which would be trialled against the disease under investigation. If a molecule showed promise, then related molecules would be synthesised until one was developed that optimised the balance between therapeutic response, unwanted side effects and cost. Sternbach's initial work in Nutley on anti-anxiety drugs using this approach was unsuccessful and, perhaps in response to this, he decided to re-examine some of the many chemicals he had prepared in Krakow in the hope that one of these might be the desired lead compound.
Dziewonski, his chief, was preoccupied with the chemistry of acenaphthalene, a somewhat obscure polycyclic hydrocarbon, but occasionally made forays into quinoline chemistry. Most of the products from Sternbach's research were nitrogen-containing heterocyclics, so there is a slight connection here. We suspect that he was simply given a chemical problem to investigate and, as a PhD student and post-doctoral fellow, he was expected to see what he might get out of it. The reaction between benzoyl chloride and 1-naphthylamine was known to give 1-benzoyl-aminonaphthalene by substituting PhCO for one of the amino hydrogen atoms.
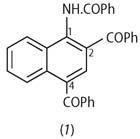
Sternbach decided to use the more forcing conditions of the Friedel-Crafts reaction to see if the benzoyl group might further substitute in the aromatic rings.[4] The basic reaction had been known since 1877 and the use of AlCl3, FeCl3 and ZnCl2 catalysts was well documented. Using this last agent, Sternbach found that the 'forcing conditions' persuaded the -COPh group to substitute, additionally, in the 4-position and, subsequently, into the 2-position (see structure (1)).
Sternbach was clearly intrigued by the ortho -substitution because he spotted that it might be possible to bridge across the 1- and 2-positions. He spent much time producing a sequence of molecules with no complicating benzoyl substitution in the 4-position. Crucially, he discovered the ring closure shown in Scheme 1.[4] This type of reaction was not new. A similar ring closure using acyl, rather than benzoyl, substituents on a phenyl ring had been reported by Karl Auwers in 1891. There was, briefly, some doubt as to the nature of the product, with Auwers favouring a five-membered ring. The notion of the seven-membered closure product was proposed by August Bischler and accepted for almost the next 70 years.
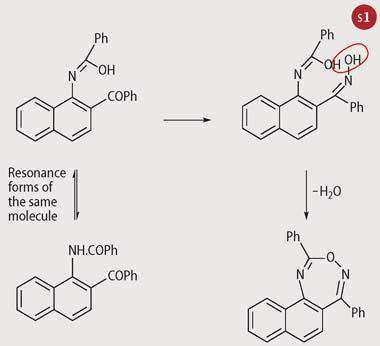
So, at Hoffmann-La Roche US in the mid-1950s, Sternbach prepared some 40 or so versions of his 'hept-1,2,6-oxodiazine' for pharmacological testing. He went on to incorporate a chlorine atom on the aromatic nucleus because this was a notable feature of chlorpromazine and several other psychotropic drugs, and introduced a tertiary amine function. The latter grouping is often associated with species such as the alkaloids, chemicals with biological action, and Sternbach was possibly hoping the group might exert a useful pharmacological influence here (Scheme 2).
Sternbach cleans up
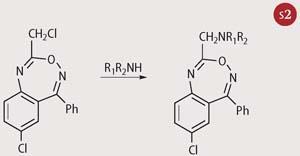
Disappointingly, the sought after biological activity was still absent and Sternbach abandoned the work. However, two years later, in a periodic lab clean-up, he noticed that one of the derivatives had been overlooked and not sent for pharmacological testing. With no real expectation of success, particularly because it was a secondary rather than tertiary amino derivative, he sent the compound to Lowell Randall, director of Roche's pharmacological department. Sternbach continues the story:
Within a few days this compound was found to possess very pronounced pharmacological properties coupled with very low toxicity. It had a particularly interesting spectrum in tests indicating central nervous system depressant activity.. These promising properties prompted the synthesis of many closely related compounds none of which, however, was significantly superior to the first member of the series.[5]
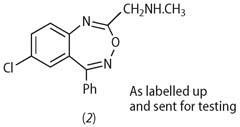
What was this compound of such promise? It had been made simply by treating the -CH2Cl compound with methylamine to give structure (2).
Meanwhile, Sternbach was wondering whether Bischler's seven-membered ring structure was correct. The action of phosphorus trichloride was particularly perplexing. As shown in Schemes 1 and 2, the oxygen atom which is part of the ring should not be particularly reactive. Yet PCl3 rapidly abstracted the oxygen to form POCl3.
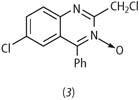
Moreover, the infrared spectra of these compounds showed a strong band at 1290-1318 cm-1, attributable to an exocyclic N-O stretching vibration. Sternbach concluded that the accepted structure was incorrect, and the 'hept-1,2,6-oxodiazines' were, in fact, N -oxides and should be renamed as quinazoline-3-oxides.[6] Thus the structure of the starting material in Scheme 2 should now be written as structure (3).
However, this was not the end of the structural story. The infrared and ultraviolet spectra of the methylamino derivative showed discrepancies from those which were expected[7] and this provoked Sternbach to do more chemical investigations. He concluded:
In addition, I found that the reaction of the (chloromethyl) starting material with methylamine did not give the normal substitution product: quite unexpectedly, ring enlargement took place to give a 1,4-benzodiazepine structure.[5]
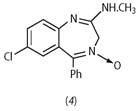
Remembering this earlier work, he revised his original request form for pharmacological evaluation to incorporate the new structure (4).
Clinical trials began in 1958 using relatively high doses, mainly on institutionalised elderly patients. It had a sedating effect and induced slurred speech and ataxia (an unsteady gait).
Apart from its relative lack of toxicity, the 1,4-benzodiazepine structure showed no particular advantages over, say, the barbiturates. Fortuitously, despite this, Dr Irvin Cohen, a psychiatrist working in Galveston, Texas, and two other practitioners agreed to trial it, in lower doses, on some of their out-patients suffering from psychoneurotic disorders (ie anxiety and less severe depression). Roche neuropharmacologist Willy Haefely describes the results:
A high rate of improvement of symptoms associated with anxiety and tension was found. Sleep was improved. In spite of obvious uncertainties about the optimal dose, the side effects were mild... and (could be) avoided by adjustment of doses. Increase of appetite, interest in social activity and verbal productivity as well as a feeling of well-being suggested some form of psychostimulant effect. The interest of clinicians in chlordiazepoxide grew enormously and, within a time which appears incredibly short today, experience with thousands of patients accumulated. The compound was introduced in the USA in February 1960 under the trade-name Librium.. The rapid onset of a therapeutic effect in low doses with only minor side effects convinced physicians and patients alike and, perhaps with the suggestive trade-name, led within a very short time to an enthusiastic acceptance of chlordiazepoxide which surpassed the most optimistic predictions.[8]
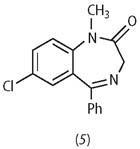
The striking success of this drug led Sternbach and Roche to pursue an intensive investigation into the benzodiazepines series to see if variations on the Librium theme might produce yet more effective anti-anxiety drugs. Somewhat surprisingly, neither the N -oxide nor the exocyclic methylamino functions were prerequisites for activity. One of the first to be sent off for testing was the relatively simple compound (5).
This species, diazepam, entered medical practice in 1963 and was given the trade name Valium. More potent than its predecessor, it has largely supplanted Librium, though both are still available on prescription in the UK. Librium's name connects with the final syllables in 'equilibrium'. Valium comes from the Latin, valere, meaning 'to be strong'.
Mother's little helpers?
The initial reaction to the benzodiazepines was extraordinary. They rapidly replaced most other sedatives and anxiolytics, and became widely prescribed for a range of other problems (irrespective of a lack of evidence for some of these uses). Ominously, they also became used as recreational drugs. The song Mother's little helpers by the Rolling Stones, released in 1966, was a reference to this widespread prescribing and abuse (and a cynical commentary on this 'sanctioned' drug abuse in contrast to the denigration of the band members' own illicit substance abuse). Within a few years their potential to induce tolerance (so that a larger dose was required to produce the same effect as before for an individual) and thus dependence, was evident. This led to problems when patients stopped treatment after many months or years, because the patient (and doctor) could confuse the anxiety symptoms of too rapid discontinuation (precipitating a withdrawal reaction) with that of re-emergence of the original symptoms and thus 'evidence' that the drug should be continued. More severe withdrawal could cause vomiting, and sweating, abdominal and muscle cramps, tremors and even convulsions. Later an even more potent benzodiazepine, Rohypnol, became notorious as the 'date-rape drug'.
While management of anxiety disorders remains challenging, the emergence of effective short-term psychological treatments and recognition of the place of antidepressant medication in treating some anxiety conditions has reduced the use of benzodiazepines. They are still used to help sleep, as muscle relaxants and as anticonvulsants in some forms of epilepsy, but the risks of potential tolerance and dependence are now better recognised. The search for more effective agents with a lower risk of dependence continues, but it would seem that every anti-anxiety medication has had an intrinsic risk of tolerance and dependence - from alcohol onwards.
Alan Dronsfield is emeritus professor of the history of science in the school of education, health and sciences, at the University of Derby, Kedleston Road, Derby DE22 1GB. Peter Ellis is professor of psychological medicine at the School of Medicine and Health Sciences, University of Otago, Wellington, PO Box 7343, Wellington South, New Zealand.
Leo Sternbach - a brief biography[9]
Leo Henryk Sternbach was born in 1908 of a Polish father and Hungarian mother in the small town of Abbazia, now part of Croatia. His father was a pharmacist and moved with his family to Krakow, Poland, to open a pharmacy there. Leo studied pharmacy and qualified, at masters level, at the University of Krakow in 1929. He was awarded a PhD in organic chemistry in 1931. He did post-doctoral work with his former supervisor, Karol Dziewonski, and in Switzerland with Leopold Ruzicka. In 1940 he was appointed senior research chemist with Hoffmann-La Roche, initially in Basle, Switzerland. By now he and his wife had Swiss passports and could emigrate (via German-occupied France, and Lisbon) to the US. He stayed with Roche for the remainder of his working life, and published 128 papers and monographs, and took out 241 patents. He formally retired in 1973, but continued to work for Roche until 2003. Reflecting on his long career Sternbach said:
It has brought me great comfort to know that I could, in some way, help people feel better. Being a chemist, I spent most of my life working in a laboratory, hoping that I could make a difference. Knowing that Valium has positively impacted the lives of millions of Americans, and that my research has paved the way for other discoveries, is one of the most rewarding experiences of my life.[3]
He died on 28 September 2005, at the age of 97.
References
[1]Panic attacks: entry on About.com website
[2]A. T. Dronsfield and P. M. Ellis, Educ. Chem., 2006, 43 (3), 74.
[3]Leo Sternbach: Roche Pharmaceuticals website
[4]K. Dziewonski and L. Sternbach, Bull. Int. Acad. Pol., 1933A, 416.
[5]L. Sternbach, Agents and Actions, 1972, 2, 193.
[6]L. Sternbach, S. Kaiser and E. Reeder, J. Am. Chem. Soc., 1960, 82, 475.
[7]L. Sternbach and E. Reeder, J. Org. Chem., 1961, 26, 1111.







No comments yet