Around the world crop fields are stripped by locusts; barley is choked by weeds; stored grain is a feast for rats, and the remainder goes mouldy. In the UK slimy molluscs destroy lettuce patches with their nocturnal munching and children get itchy heads from egg-laying lice. The main weapons against such pests are now synthetic organic pesticides.
-
Production of food crops is heavily dependent upon organic synthetic pesticides
-
Modern pesticides are more selective and more potent than earlier chemicals used, so less is required

Pesticides are big business. A global industry worth around £20,000 m, there are over 700 chemicals in use as pesticides, which are formulated into around 35,000 products - insecticides, herbicides, fungicides and rodenticides. World consumption of pesticides is 2,000,000 tonnes which divides up as: US, 24 per cent; Western Europe, 28 per cent; Far East, 25 per cent; Eastern Europe, 8 per cent; and the rest of world, 15 per cent. Recently, however, there has been a decrease in the amounts of pesticides used owing to the identification of new active ingredients, which are more potent and required in smaller doses. What are these pesticides and how do they work?
The first synthetic insecticides
Before modern synthetic organic pesticides became available inorganic chemicals, such as copper acetoarsenite (Paris green), lead arsenate and sodium fluoroaluminate (cryolite), were applied to crops to kill insects. The main organic insecticides were the natural pyrethroids, extracted from chrysanthemums, but these were expensive and available in only small quantities.
The modern, synthetic, pesticide industry began when the insecticidal properties of DDT (dichloro-diphenyltrichloroethane, 1) - an organochlorine compound known since the 1870s - were discovered in 1939 by Paul Müller, earning him the 1948 Nobel prize in medicine. Since then the number of compounds with insecticidal properties has grown enormously.
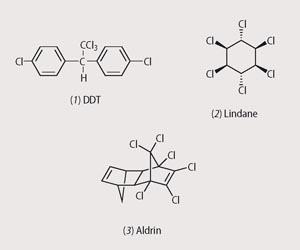
Around two million tonnes of DDT were used worldwide between its introduction in 1940 and phasing out in the early 1970s. Its greatest success was in the control of insect-vectored diseases such as malaria and yellow fever between 1942 and 1952 - the use of DDT is believed to have saved five million lives and protected many millions from illness. DDT destroys the balance of sodium and potassium ions within the nerve cells (neurons) so that transmission of normal nerve impulses is disrupted. Affected insects twitch with what is known as the 'DDT jitters', leading to convulsions and death.
Other highly effective organo-chlorine insecticides followed. Notable examples were hexachloro-cyclohexane isomers, for example lindane (2), discovered in 1940 by British and French entomologists and, at the end of World War II, the chlorinated cyclodienes, such as aldrin (3), came on the market. The latter was particularly effective against the insect larvae that devour the roots of plants.
Organochlorine insecticides are stable and persist in the ground for a long time - DDT has a half-life of up to 10 years. This, along with the fact that the organochlorine compounds are highly soluble in body fats and can move with relative ease through the food chain, presents significant environmental and health concerns.
In the 1970s aldrin and related compounds were withdrawn from use in many countries. Soon after other organochlorine insecticides fell from favour as evidence of bioaccumulation and their movement in the food chain grew. Later, there were also worries that these compounds might be potential carcinogens. Despite these problems, however, the organochlorine insecticides had a major impact upon insect control and demonstrated the contribution that synthetic organic chemistry could make.
Organophosphates
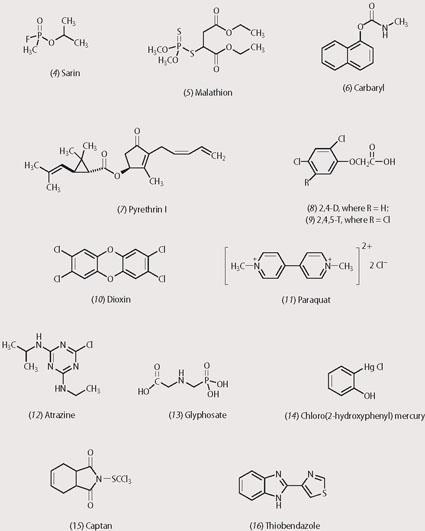
During World War II, phosphorus-based compounds - organo-phosphates and organophosphorus compounds - were receiving a lot of attention from German chemists. Organophosphates were showing promise as insecticides, while organophosphorus compounds, eg sarin (4), were found to be effective against humans as well as insects.
Organophosphates are esters of the phosphorous acids and as such the phosphorus atom is bonded to oxygen and not directly to carbon as is the case with organophosphorus molecules. The difference is subtle but it has a profound effect upon reactivity. Organophosphates attack the nervous systems of both insects and humans but in no way are they as damaging as the organophosphorus compounds, which were put to use as nerve gases in chemical warfare.
Malathion (5) is an organo-phosphate insecticide in use today. This compound inhibits the enzyme cholinesterase, which breaks down the neurotransmitter acetylcholine into choline and ethanoic acid. Malathion phosphorylates the enzyme, giving a stable product. Thus, acetylcholine is not broken down and accumulates in the neurons, where it destroys the normal workings of the central nervous system which leads to death. Malathion is highly toxic to insects but is relatively harmless to humans because we have enzymes that hydrolyse the molecule to detoxify it. In fact malathion is used in hair preparations for killing head lice.
Over the years many organo-phosphates were developed but several had to be withdrawn because they were toxic to humans. Generally though organophosphate insecticides are biodegraded and so, unlike the organochlorine compounds, they do not accumulate in the body. Those in use today are the less harmful ones but they still pose a risk and handling procedures need to be strictly complied with.
Other insecticides
The most recent chemical weapon in the fight against insects are the carbamates, which came into use soon after World War II. These compounds are less toxic to humans than either the organochlorines or the organophosphates. Carbaryl (6), in particular, offers low mammalian toxicity while at the same time being powerful enough to kill a wide spectrum of insects. The low human toxicity is a result of the speed with which the carbamate is detoxified and excreted in the urine, thus preventing its accumulation in body fats. Carbamates can therefore be applied to vegetables and fruit crops and used on farm animals to kill parasites. Carbaryl, like the organophosphates, inhibits the enzyme cholinesterase.
Pyrethrins are highly effective insecticides. Pyrethrin I (7), the active ingredient, is extracted from chrysanthemum flowers (Chrysanthemum cinerariaefolium) by solvent extraction. Pyrethroids penetrate the insect's cuticle with ease and their subsequent toxicity depends upon them disrupting the nervous system in a mechanism similar to that of DDT.
The first synthetic pyrethrins were made in the 1940s and since then a whole series of compounds has emerged with cypermethrin being one of the most important in the class. These insecticides are capable of killing a broad spectrum of insects and are effective at application rates as low as 5-10 per cent of those of other insecticides. Furthermore, they remain active in the soil for up to four weeks whereas their botanical counterparts last for only a day or so. Unfortunately, pyrethroids are toxic to fish and to some beneficial insects.
There are several other groups of insecticides and many examples within each group. Most are applied as powders, solutions or dispersions. There is occasionally the need for volatile compounds, eg methyl bromide, to be used as fumigants to permeate large volumes of materials such as stored grain or fruit in warehouses. The use of methyl bromide, however, is now in decline owing to environmental concerns.
Other approaches to prevent insect damage include the use of synthetic insect sex pheromones to disrupt mating and reproduction.
Herbicides
In early times, when agriculture was less intensive, weeds were controlled by physical methods such as mowing, cultivating, burning and crop rotation. As populations grew, demands upon the farmer increased and methods requiring less labour were called for. This paved the way to wide-scale use of chemicals for weed control. Examples included borax, arsenic trioxide, carbon disulfide, sodium chlorate and sulfuric acid.
From 1945, as with insecticides, synthetic organic chemistry became the foundation of modern weedkillers. Thanks to research, we now have effective herbicides that can be applied at a fraction of the dosage rates of the early chemicals. Herbicides may be either selective for a particular plant species or non-selective if they kill plants in general.
Phenoxys
The most widely used weedkiller is 2,4-D (8), which works by mimicking and disrupting the action of auxins - plant hormones that control the growth of shoots. 2,4-D was developed by British chemists during World War II and became the first commercial herbicide for control of broad-leaf weeds. The related compound, 2,4,5-T (9) was used to control woody perennials. In fact, 2,4,5-T, which became known as Agent Orange, was used by the Americans in the Vietnam war to remove the jungle cover by defoliating the forest and to destroy the enemy's food supply by killing the rice crops. The dosage rates were many times higher than those permitted for normal agricultural use. There are now allegations that some of the 2,4,5-T used in the defoliant contained the lethal byproduct dioxin - 2,3,7,8-tetrachlorodibenzo-p-dioxin (10). Dioxin, one of the most toxic substances known, biodegrades slowly and remains in the environment, and in living tissue into which it has been absorbed, for a long time. Exposure of military personnel to Agent Orange, and to dioxin, is blamed for the health problems that many veterans subsequently experienced.
Bipyridiniums
The bipyridinium, paraquat (11) has been used on a large scale in aerial sprays to destroy cannabis and coca plants. Although highly effective as a non-selective contact herbicide, this compound causes irreversible lung damage and its ingestion can be fatal. Thus, despite its effectiveness as a weedkiller paraquat ranks as the most toxic of commercial herbicides. Paraquat damages the anti-oxidative system that protects plant cells from free radical damage. The radicals build up and produce oxygen which then goes on to attack the cell membranes, causing them to rupture.
Triazines
Atrazine (12) is a widely used selective weedkiller for controlling broad-leaf weeds and grasses in, for example, cornfields. It is a low cost herbicide and can have a synergic effect with other herbicides, resulting in improved activity and reduced application rates. Atrazine interferes with the photosynthetic mechanism so that CO2 fixation is prevented. The growth of the weed is hindered, which makes it vulnerable to other destructive processes.
Amino acid derivatives
Glyphosate (13) is a non-selective broad spectrum herbicide used to control grass and broad-leaf weeds. Glyphosate inhibits the enolpyruvylshikimate enzyme involved in aromatic amino acid formation. The amino acid derivative can be used for weed control in genetically modified crops which have been modified to resist its effects.
Fungicides
Early chemical methods - a few of which are still in use today - for preventing fungal attack upon foliage relied on dusting with flowers of sulfur, London purple (arsenic trioxide, aniline, lime and ferrous oxide) and Bordeaux mixture (calcium hydroxide and copper sulfate). Organometallic compounds have also played a role as seed fungicides. Even mercury compounds, such as chloro(2-hydroxyphenyl) mercury (14), have been used but in most countries these are now banned because they are very toxic.
Of the modern synthetic fungicides, captan (15) is popular. It has a broad spectrum of activity which derives its toxic action by reacting with thiol groups. The reaction releases thiophosgene which disrupts essential enzyme chemistry of the fungi. Thiobendazole (16), another modern fungicide, exerts its toxic effect by disrupting the polymerisation reactions essential to the cyto-skeleton of the fungi.
Rodenticides
Some highly toxic chemicals are on the list for killing rats and mice. Yellow phosphorus paste, white arsenic and strychnine (17) have all been used in the past. Rodents that have ingested the phosphorus produce phosphorescent smoking faeces prior to death.
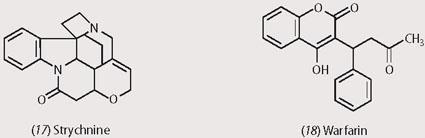
In around 1940 warfarin (18) was found to be toxic to animals - cattle died after eating sweet clover which contains the chemical. Warfarin, a derivative of coumarin, is active at as little as 0.025 per cent in the bait used and, being a vitamin K antagonist, reduces blood clotting which results in internal bleeding.
A problem with rodenticides is that they are non-selective and other animals are often accidentally poisoned. An exception is red squill, a botanical preparation, which kills rodents but is harmless to other animals - and children - because, once swallowed, the squill is immediately vomited out. Rodents are unable to vomit and so the poison is retained.
Pesticide resistance
Modern pesticides are highly effective but despite this they seldom achieve a 100 per cent kill of the particular pest. This is particularly noticeable with insects - after being subjected to a dose of pesticide, there are nearly always some insects that survive the attack.
This is partly because some members of the population have advantageous genetic characteristics. For example, some insects will have inherited just the right biochemistry to enable them to detoxify the pesticide fast enough to prevent it killing them. The remaining insects, those in which this inherited characteristic is absent, are unable to detoxify the pesticide quickly enough and so are killed by it. The net effect is that those resistant to the pesticide go on to breed and pass on the characteristic. Their offspring and future generations will contain fewer vulnerable individuals and so a resistant population evolves.
This resistance was noticed with the first synthetic insecticides. For instance, the first applications of DDT gave rapid results. But it was soon found, that with repeated usage, the chemical was having less effect. Application rates had to be increased to get the same results as in initial treatments. It later became clear that the rapid reproductive rate of insects enabled a new generation every few weeks which favours quick adaptability to new threats.
Pests that have become resistant to one particular compound are often seen to be resistant to other compounds of the same class (class resistance) which work by a common mode of action. The development of new classes of pesticides with different modes of action is one way to combat this problem.
As Nature fights back she is providing the pesticide chemists with plenty of challenges which will, no doubt, introduce new and exciting areas of chemistry.
Dr Tony Hargreaves is a chemical technologist and can be contacted at 55 Heather Road, Meltham, Holmfirth, Yorks HD9 4HT (e-mail: AHargreaves66574@aol.com).
Further Reading
- Agricultural chemicals and the environment, issues in environmental science and technology, R. E. Hester and R. M. Harrison (eds). Cambridge: RSC, 1996.
- The pesticide manual, C. R. Worthing (ed). Farnham: British Crop Protection Council, 1994.
- Pesticides chemicals and health, The BMA guide to, D. R. Morgan (ed). London: BMA, 1992.
- Pesticides - developments, impacts and controls, G. A. Best and A. D. Ruthven (eds). Cambridge: RSC, 1995.
- The pesticide index, L. G. Copping, H. Kidd and C. D. S. Tomlin (eds), 3rd edn. Farnham: British Crop Protection Council, 1995.
- Encyclopaedia of chemical technology, Vols 12, 13, 14 and 18, 4th edn. Chichester: Wiley, 1997.






No comments yet