Investigations involving simple batteries made from items found in the home or school laboratory can help KS3 pupils understand the origin of current, voltage and power, and the chemistry that drives batteries.
Rough science is the Open University's popular science programme on BBC2 in which five scientists are set scientific challenges, which they have to complete using natural resources. Inspired by this series, investigations involving simple batteries made from items found in the home or school laboratory can help KS3 pupils understand the origin of current, voltage and power, and the chemistry that drives batteries.
A simple battery can be made by pushing a screw (best if it is zinc-coated/galvanised) and a piece of copper into a lemon and connecting these two electrodes using wires to the device you want to power. This cell can produce enough power to run an LCD clock/watch. Using a carbon-rod electrode instead of the copper also works, and you can use potatoes as well as lemons. Three potatoes, each having a carbon rod and a galvanised screw electrode system can be wired up and will supply enough power to light a light emitting diode (LED). How a pair of electrodes produces a voltage when immersed in an electrolyte is revisited in the Box.
Sea-water batteries
In the first series of Rough science, broadcast in 2000, set on a remote island off the coast of Tuscany, we had to make a sea-water battery. We used galvanised (zinc-plated) screws and carbon rods as the electrodes, and saltwater as the electrolyte. While carbon is a good conductor of electricity, the chemistry that takes place at the carbon electrode is slightly different than would be the case when simply using metals. However, slightly larger potentials are produced using carbon and zinc than with copper and zinc. In such cells the metal (zinc) electrode is negative and the carbon electrode is positive.

To get round the fact that the voltage and current of such a simple cell is limited, we wired up several cells in such a way as to combine the output. One effective arrangement is shown in Fig 1. We used a 12-compartment ice-cube tray to hold the saltwater electrolyte, and a wooden frame support for the 12 pairs of (zinc and carbon) electrodes. We found that 'carbon rods' are much better than 'graphite rods' of the same high purity (even though they are both made of carbon). Carbon rods appear to be more porous than the shiny graphite rods. They therefore present a much greater surface area to the electrolyte. As a consequence, the electrode resistance is much lower, creating a better battery. To make a similar battery in the lab you will need:
- tap water;
- table salt (NaCl);
- 12-compartment (or similar) plastic ice-cube tray;
- piece of wood or thick card to support the electrodes; tinned copper wire (ie with no insulation);
- 12 galvanised screws (ca 5cm long), one for each cell, obtainable from any hardware store;
- 12 pencil leads (2B or softer), one for each cell, or you could use school laboratory 'carbon' rods, or salvage them by carefully dismantling old batteries.
Experimental
Make up a dilute solution for the electrolyte by dissolving a teaspoon of salt in a mug of water (or if possible you could use sea water instead). Fill each of the ice-cube tray compartments three-quarters full with the salt solution. Lower the electrode pairs (one of zinc and one of carbon) into their corresponding ice-cube tray compartments to create the 12 cells. To complete the battery the electrodes will need to be wired up on the top side of the wooden support.
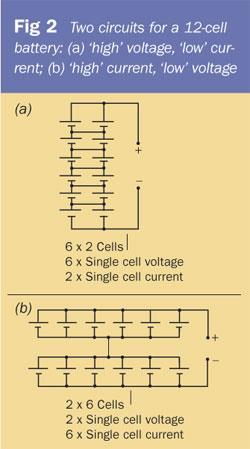
There are several ways of wiring up the cells. Combinations of series and parallel-wired cells will produce a range of voltages and currents. The arrangement in Fig 2 (a) consists of two sets of six cells wired in series, with these two sets then wired in parallel. Such a combination would produce a total of 6 × V volts and 2 × I amps (where V and I are the voltage and current, respectively, produced by a single cell). The arrangement in Fig 2 (b) consists of two sets of six parallel-wired cells, each set wired in series. Such an arrangement generates a total of 2 × V volts and 6 × I amps. The battery circuit in Fig 2 (a) provides a relatively higher voltage than the circuit in Fig 2 (b), and it can therefore be used to power devices that need 'high' voltages but 'low' currents - a pocket LCD calculator, an LED (and series resistor), or possibly even a pocket radio, will work well using this arrangement. (Note: in circuit Fig 2 (a) you don't need the central horizontal connections between the pairs of cells for the battery to work but it does make the overall battery less prone to any bad connections there may be between the cells.)
The battery circuit in Fig 2 (b) will work well for devices that need a 'high' current but not a particularly large voltage. A good example would be a low-voltage 'solar cell' motor that will run on about 1 V, but needs >10 mA to turn. Figure 3 shows a flashing LED circuit wired to the battery. This circuit requires about 3 V, at only 1 or 2 mA, to work.

The basic sea-water battery can drive a number of low-power devices. However, since the battery is converting chemical potential energy into electrical energy the battery will eventually fail (run down) as the chemistry at the electrode surfaces and in the electrolyte develops. The zinc on the galvanised screws dissolves over time, and zinc hydroxide may also form on the electrodes, thereby increasing the resistance. The electrodes therefore need to be cleaned each time you use the battery, so as to reduce the chemical build-up that inevitably occurs.
Further activities
Students could use different electrode materials to assess the range of voltages and currents that can be obtained. They could also see what effect changing the surface area of the electrodes has, and what happens when they use different electrolytes (try lemon juice or vinegar, for example). Other experiments would be to find out if the current produced by the battery depends on electrolyte concentration, and does the battery fail if you over-dilute or over-strengthen the concentration? Would an increase or decrease in electrolyte temperature affect the voltage and/or current produced, and if so, why? Does this help to explain why you can temporarily rejuvenate used batteries by putting them on a warm radiator?
In a recent series of Rough science, filmed in Zanzibar (shown on BBC2 in March 2005), we were challenged to make an emergency life jacket incorporating LEDs that would be activated and light on contact with sea water. We simply modified the ice-cube tray sea-water battery by placing small pieces of sponge between the electrodes in each of the tray compartments. When the life jacket went into the sea, the individual sponges soaked up sea water, thereby completing an electrical circuit and cell chemistry in each cell. The current that was produced was then used to power several LEDs.
The Baghdad battery
In 1800, Count Alessandro Volta made what was thought to be the first device that approximated to a modern battery. He found from experiments that different metals in contact with each other via paper strips soaked in saltwater generated electricity. He built a stack of alternating discs of zinc and silver, separated by pieces of blotting paper soaked in saltwater. When he attached a wire to the top and bottom discs he found he could measure a voltage and a current. This device became known as a 'voltaic pile'. But was Volta's pile the first battery? Some people believe that batteries have been around for more than 2000 years. Their 'evidence' comes from Baghdad.
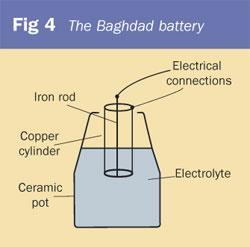
In 1938, Von Wilhelm Konig, an archaeologist working at the Baghdad Iraq Museum, claimed to have discovered a much older battery than Volta's. Konig claimed that it was made by the Mesopotamians as long ago as 200BC. His 'Baghdad battery', shown in Fig 4, consisted of a ceramic pot about 14 cm high, in which was placed a cylinder of copper encasing an iron rod. The iron rod was surrounded by an oil/pitch-based insulator to separate the two. In the pot, Konig found mysterious evidence of organic material.
We recently made a copy of a Baghdad battery. Our device used the same electrodes as the original, with vinegar (which would have been available at the time) as the electrolyte. Our Baghdad battery produced about 1V at 10mA. Such a system did indeed work as a battery, but was this the Mesopotamians' intention over 2000 years ago - or did the original device have some other, quite unconnected purpose?
It seems unlikely to us that the Mesopotamians intended to make a battery; an observation supported by the fact that no wires were found with the device (though these could easily have rusted away over time). On the other hand, if they were using them as batteries, what might their purpose have been? One suggestion is that the Mesopotamians may have wired up many such batteries so that they could electrify holy statues - to give them seemingly supernatural powers. This is a nice idea, but electric shocks depend on high voltages, and hundreds of batteries would have been needed to produce anything approaching an impressive shock.
Another theory is that the devices were used to produce small voltages that could have been used in acupuncture (a therapy in use at the time). Indeed, some of the batteries were actually found next to fine needles. It is possible that needles applied to the skin and wired so as to generate a few volts might have produced a therapeutic effect.
In the course of our own recent investigations, we have used the voltage from several Baghdad batteries wired up in series to drive a simple electrolysis reaction. The idea was to electroplate some copper metal using a cell containing vinegar, silver and copper electrodes. Powered by the Baghdad batteries, silver ions are produced at the silver electrode (we used a silver bracelet), which then travel through solution to form a thin layer of silver metal on the (cheaper) copper electrode. Given enough time, we found we could successfully silver-plate the copper.
Could the ancient Mesopotamians have used a similar process to plate intricately designed copper statues with a layer of silver? Unfortunately, no proof for this wonderfully appealing possibility has yet been found in the archaeological records. The origin, use and purpose of Baghdad batteries remains a mystery.
(Note: many companies that sell electronic components stock 'ultra bright' LEDs as well as 'solar-cell' motors that work on very low voltages and currents. These would be suitable for use with the experimental batteries described in this article.)
Acknowledgments: we thank NESTA, the BBC and the Open University, and in particular, David Shulman, Paul Manners, and all the 'Rough Scientists'. Many thanks to Graham Riley for valuable comments, and also to Cicada films and Professor Tony Ryan for work on the Baghdad battery.
Dr Mike Bullivant is retired but can be contacted at The Open University, Walton Hall, Milton Keynes MK7 6AA, and Dr Jonathan Hare runs the Creative Science Centre, University of Sussex, Falmer, Brighton BN1 9QJ.
Batteries revisited
When a metal is immersed in an electrolyte, positive metal ions are formed at the surface of the electrode. These ions pass into solution, making the electrode more negative as the positive ions move away. There will come a time, however, when the resulting, negatively charged electrode will start to attract back the oppositely charged metal ions. A dynamic equilibrium is eventually set up between those ions leaving and those returning to the metal surface. How far the equilibrium goes one way or the other depends on the reactivity of the metal involved.
A cell has two electrodes, and a similar process takes place at the second electrode. If this metal is the same as the first, then each electrode will charge to the same extent, and there will be no resulting charge difference between the two. There will be no attractive force between them and no potential difference or voltage will be developed.
However, if the electrodes are made of different metals, different equilibria will be set up on each, with one electrode becoming charged to a greater extent than the other. Because of this charge difference, electrons will move towards the more positive electrode when a circuit is formed. This attractive potential is the voltage that we measure between the electrodes and the origin of the cell's power.
If a wire is connected between the two electrodes to form an electrical circuit potential energy (work that can be done), which the moving electrons will have once a circuit is established, is the voltage of the battery.
V = Volts = energy per charge/electron
(joules/coulomb)
The amount of charge passing per unit time is the current:
A = Amp = rate of charge passing
(coulombs/s)
Since the charge on the electron is tiny (1.6 × 10-19 C), even a current of 1/1000 Amp (1 mA, just enough to light an LED) represents a massive number of electrons moving, ca 1× 1015/s. If we multiply the voltage between the electrodes by the current that is passing, we get the power of the cell:
P = V× A
= (joules/coulomb) × (coulomb/ second)
= joules/second
The power is the amount of work done per second.
Battery limits
The voltage of the cell is determined by the difference between the individual standard electrode potentials of the two electrodes involved. However, in practice, this is only true in the ideal case in which the voltage is measured without drawing any appreciable current. This is the case when using a very high resistance voltmeter, but not the case when we use a battery to drive a light or a radio for example. In practice as we draw current from the cell we see that the voltage drops away.
This limitation of a real life battery can be attributed to at least two factors: the nature of the electrolyte and the electrical resistance of the electrodes. The dependency of cell voltage on the electrode potentials explains why very similar results can be obtained by using different electrolytes (for example, sea water, vinegar, sulphuric acid, and even urine). The cell voltage is also dependent on the concentration and type of ions in the electrolyte, the limiting case being when there are no ions present at all (eg in a non-ionic liquid or a battery that has dried out).
Assuming that the electrolyte is above a threshold concentration, the surface resistance of the electrodes also has to be considered. This is the electrical resistance made between the solid electrode and the solution (by the passage of the electrons and ions). If the electrode resistance is low, the cell will produce a nearly constant voltage as more and more current flows. If the resistance is high, the voltage will appear to drop significantly as current is drawn from the battery. The internal resistance of cells is a major limiting factor in the application and usefulness of real batteries.
Related Links
The electrochemical series
Explains the origin of the electrochemical series, and the ability of the various substances included in it to act as oxidising or reducing agents
Creative Science Centre
Two low power LED flashers
Riddle of 'Baghdad's batteries'
What could have been the very first batteries
The UnMuseum
Baghdad battery
BBC/OU Open2.net
Details on the Rough Science series
The Creative Science Centre
To stimulate enthusiasm and experience of science by providing ideas, resources and supervision






No comments yet