Public analysts are responsible for the safety and quality of our foods and check that the labels give us correct information. These highly-skilled analytical scientists also test a wide range of consumer goods as well as water and air samples that may have an effect on human health or the environment. More recently, some laboratories have been involved in the fight against terrorism and run tests on hazardous materials and suspicious powders.
-
Professional chemists use a range of analytical techniques to protect the public's interest

Have you ever wondered if your favourite tipple has been diluted with water or your preferred brand of whisky has been substituted by an inferior brand? Or who do you go to if you suspect that the food or drink you buy isn't up to standard, or worse has been contaminated? The answer is that all food and drink complaints are submitted to the public analyst within a local authority, of which there are some 400 in the UK. By law, food must not contain harmful substances, and additives in food, such as preservatives, are limited as to what and how much can be used, and there are minimum standards for the composition of some foods.
In the course of their work, public analysts will use a host of analytical techniques, microbial assays as well as physical and mechanical tests on, for example, consumer goods. They do not generally get involved in collecting samples, though there are exceptions. The latter is generally done by trading standards officers and environmental health officers. If the public analyst finds something wrong then these officers will try to get the shop or factory to change what they are doing - if they refuse or if they have tried to cheat the public in any way, then they are taken to court. All public analysts are required to understand what problems are likely to arise with any given product or situation and must be able to interpret the results of the analysis, and to be an expert witness in a court of law.
Analytical expertise
Branded spirits are sold by the measure (25ml or 35ml) and the European Union lays down the rules on what defines such drinks, specifying, for example, a minimum alcoholic strength, which for whisky is 40 per cent by volume. Any consumer complaints on 'brand swapping' or dilution are sent to the public analyst who determines the alcoholic strength of the sample by densitometry, and, for whisky, will use gas chromatography to separate and measure the concentrations of congeners. Congeners - aldehydes, esters and alcohols - are naturally occurring flavour compounds which are formed during fermentation and maturation of whisky. The various alcohol fractions and the congener 'fingerprint' are compared with those for the authentic whisky sample (see for example Figs 1 (a) and (b)).
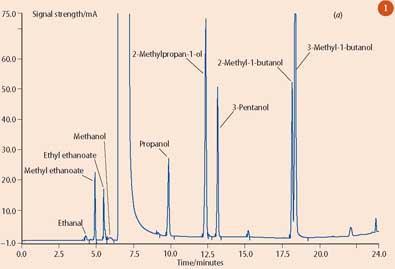
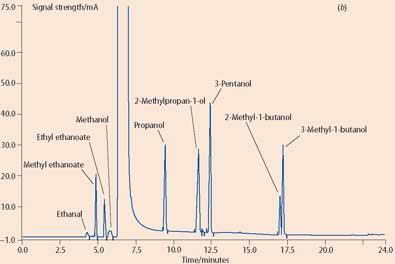
Authenticity of other spirits is determined using other 'markers', which may be naturally occurring, such as botanical extracts from gin, or some are added to protect the brand, such as trace sugar levels in vodka. As well as determining the authenticity of a spirit, analysis is tailored to detect the methanol content of counterfeit products. Occasionally spirits have been found to contain dangerous levels of methanol, which is highly toxic - ingesting just 10ml can cause permanent blindness, while 60ml can be fatal.
Sometimes you can get more than you bargained for. A recent sample of brand lemonade was submitted to a public analyst because it had made the consumer unwell. The liquid had no bubbles and was found to be vodka. The staff had been decanting the lemonade, filling the empty lemonade bottles with vodka from the shop and buying these on their way home for a fraction of the true price.
Food complaints, including the deliberate addition of harmful materials - insects, glass and chemicals - into supermarket products, are submitted to the public analyst who is expected to identify at what stage of the production or of the distribution a contaminant was introduced.
Heavy metals in foods form part of routine analyses whereby food is subjected to acid dissolution, often using microwave digestion followed by atomic absorption spectroscopy or inductively coupled plasma-optical emission spectroscopy (ICP-OES). These techniques allow a large number of samples to be tested for a wide range of heavy metals in a relatively short period of time.
Food allergies are common, so it is important that food products are adequately labelled to allow the consumer to make the correct choice when buying food. A range of immunoassay techniques are used to detect traces of peanut or to determine the presence of gluten. More recently, DNA analysis has been used to differentiate between fish and meat species, and can be used to identify certain types of rice such as basmati.
Most laboratories also provide consumer testing facilities of household chemicals, children's clothing, toys, candles and cosmetics to ensure that they are safe. Paint on toys and pencils, for example, must not contain toxic heavy metals such as lead and there are limits on the presence of harmful phthalate plasticisers.
The laboratories also support local authorities to ensure good air quality and to identify contaminated land. Water quality is checked by analysing public and private water supplies, surface, ground and bathing water. Public analysts also work with health and safety practitioners by sampling and analysing indoor air, doing specific workplace assessments or investigating problems relating to environmental conditions.
Since the 9/11 terrorist attack in the US, and subsequent suspected anthrax incidents, the UK has experienced a huge number of 'suspicious packages' and 'white powder' events. Invariably the police get involved in the investigation and in turn rely on public analyst laboratories to determine the actual nature of the material. The analysis of such unknown materials presents a number of analytical challenges, which are exacerbated when the analysis is required in a suspected contaminated zone and it is necessary to wear gas suits with breathing apparatus. While the majority of these incidents turn out to be hoaxes, the scientific adviser must be able to show that the sample is free from biological material, radiological contaminations and chemical components that could be harmful to health. Once this has been established, further analysis is required as part of the police investigation to determine what the substance is. The on-scene scientific adviser will use a range of techniques such as low- and high-powered microscopy with appropriate staining techniques, spot tests for a range of anions and cations, an assessment of the physical properties of the substance as well as infrared spectroscopy and where appropriate headspace GC-MS.

The substance could also be a mixture. One disgruntled employee of a food manufacturer planned to contaminate biscuit material with a white powder. Fortunately this was discovered before the contamination occurred and the sample was sent to the public analyst lab for examination. A visual examination showed that the powder was a white, uniform crystalline material. However, when viewed under a low-powered microscope, it was obvious that the powder was a two-component mixture, with distinct crystal shapes. The scientific adviser then separated the two components physically and analysed them separately. The mixture was found to contain sodium chloride and sodium chlorate. This analysis would have been more challenging if the mixture had not been separated and because many of the tests done on site use very small quantities of material, this increases the risk of one of the two components being missed.
Career prospects

There are 22 public analyst laboratories throughout the UK each employing between 10 and 100 staff, with around 35 fully qualified and appointed public analysts.
These laboratories recruit people at all levels, from school leavers with GCSEs, A-levels and HNCs to graduates, depending on the size of the particular laboratory and the needs at any one time. In view of the small size and personal service provided by these laboratories there is considerable scope in the service for the career chemist.
Public analysts all have an honours degree in chemistry as well as a masters in chemical analysis (MChemA), one of the highest qualifications in analytical chemistry in the UK. The latter is acquired after at least three years' experience in an appropriate laboratory. There are no specific courses available to cover the whole syllabus, though the Association of Public Analysts runs a short residential course each year which is targeted at providing relevant input. MChemA holders are in short supply and are well equipped to work both in enforcement and in industry.
Gary Walker is the public analyst at Glasgow City Council, Glasgow G21 1XG.
The suspicious suitcase

A gentleman entered an up-market, city centre hotel one evening pulling a suitcase, and tried to negotiate an acceptable price for a room for the night. Following much negotiation, he left reception and was caught on video camera in a number of locations within the hotel before abandoning the suitcase in the hotel bar. The staff became concerned and called the police. Owing to the suspicious circumstances relating to this incident, the fire service was called together with a scientific adviser from the public analyst laboratory.
Wearing suitable protective equipment, and after the police were satisfied that the suitcase did not contain an explosive device, the scientific adviser inspected the case and its contents. Contained within the case was as a 10-litre white plastic drum, spray-painted gold, wrapped in cling film with three of the four upper edges cut away. A small amount of whisky-coloured, clear liquid could be seen within the drum. The area was screened for radiation before a sample was removed to the mobile laboratory. Using a meter to measure α, β, γ and x-rays, the scientific adviser established the absence of radioactive materials.
Only a few millilitres of liquid were available for testing and this was carefully removed to the mobile unit. Using a portable FTIR instrument, the scientist obtained an infrared spectrum and compared this to one in the instrument library. There was little infrared activity and the predominant peaks were assigned to the presence of water. The pH of the liquid was near neutral. The adviser then did several spot tests to check for the presence of anions and cations, which also proved negative, as well as checking for the presence of spores using high powered microscopy.
The scientific adviser took the remaining liquid back to the main laboratory where trace metals analysis was done using ICP-OES. The presence of trace levels of anions was established using ion chromatography. The liquid turned out to be predominantly water with a trace amount of detergent. The mysterious gentleman was never found and the presence of this strange package contained within a suitcase remains a mystery. However, the on-site examination backed up with trace analysis in the main laboratory showed that the liquid was harmless.
Environmental concern

Over a period of at least seven years, a number of staff at a primary school had been diagnosed as having breast cancer. Staff were concerned that the environmental conditions contributed towards the disease. The public analyst laboratory was requested by environmental health and health protection to come up with a comprehensive survey to determine whether environmental conditions in and around the school were a contributing factor. A total of 118 samples were obtained and analysed during the course of this investigation.
Soils samples from an allotment within the school grounds, and samples of street dust from around the school were analysed for potential carcinogens - including pesticides, polycyclic aromatic hydrocarbons, polychlorinated biphenyls and heavy metals.
Water samples were taken from all sources within the building and tested for the full range of chemical and bacteriological parameters specified in the drinking water regulations.
Air samples from in and around the school were analysed for the presence of man-made ionising radiation, and magnetic and electric field strength measurements were determined.
The chemicals considered as being potential links to breast cancer were either not detected in the various sample types, or were present at insignificant concentrations.
Nuisance odours
Following repeated complaints of odours from a takeaway shop below a flat, the laboratory was requested to investigate. Using tenax tubes and air sampling pumps, an environmental health officer took samples of the atmosphere within the flat and sent them to the public analyst, who did a detailed analysis by gas chromatography using thermal desorption and mass spectrometry. The analyst detected many food-related volatile organic compounds, the profiles of which closely matched the atmosphere in the shop. As part of the investigation, the analyst did real-time carbon monoxide and carbon dioxide monitoring to assess whether combustion gases from the gas cookers featured in the atmosphere in the upstairs flat.
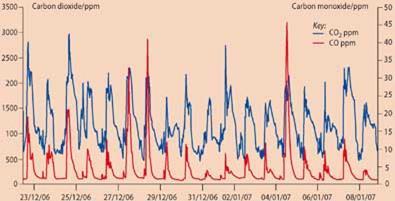
The results revealed significant levels of carbon monoxide in the flat, which was particularly alarming since the residents included a two-year old child. Furthermore, the pattern of peaks revealed that combustion gases - CO2 and CO - were present when the shop opened and disappeared when it closed.
The analyst requested a detailed ventilation assessment of the shop which revealed that the extraction system used in the shop kitchen was inadequate. Furthermore, the pots used on the cookers were far too big, changing the burning characteristics of the gas-fired rings and resulting in elevated levels of carbon monoxide. What turned out to be an investigation based on nuisance (odours) had become far more serious with regards to health and safety of the people in the flat above and the staff working in the kitchen.
A notice was served on the shop and following detailed remedial works and further monitoring, the problem was resolved. However, as a result of a number of other indoor air investigations in similar properties above takeaway shops, it became apparent that this was not an isolated case and odour complaints in these situations are now accompanied by combustion gas assessments.






No comments yet