Seven tips to craft the perfect multiple choice questions and sharpen your students’ chemistry understanding

People often see multiple choice questions (MCQs) as easy, but that really isn’t the case. There is a mistaken belief that, as the correct answer is in the provided list, students will simply be able to recognise it, rather than working through the problem. In fact, MCQs are an incredibly useful teaching tool, not just for assessment, but to strengthen student learning.
It isn’t easy to create effective questions that focus more on application of knowledge and skills than simple recall. But with careful planning, you can use MCQs to instil a deeper understanding and better long-term retention. Try these tips to help you write effective questions which challenge your students without overwhelming them.
1. Identify learning goals
Before setting MCQs, identify what you are trying to assess. This avoids selection of multiple recall-focused examples – unless, of course, that is your goal. It can be helpful to use your exam board specification to help you identify key student outcomes, and this will help you to be selective.
2. Keep it simple
Questions, also known as the stem, should be direct – avoid double negatives and use accessible language. They must be unambiguous and use clear command words which students regularly encounter. It is important you ensure there isn’t a direct copy of class notes; learners should apply their skills and understandings to reach the correct answer.
| Example question | Explanation and improved question |
|---|---|
|
Which of the following is correct? A) Atoms contain protons, neutrons and electrons B) Atoms have an overall charge C) Atoms contain protons and electrons only D) Atoms have no overall mass |
This question has no clear stem or command word; there is no information within the question to activate students’ prior knowledge. Instead, try: Which option gives the subatomic particles found inside an atom? A) Atoms contain protons, neutrons and electrons B) Atoms have an overall charge C) Atoms contain protons and electrons only D) Atoms have no overall mass |
3. Present and lay out clearly
Match the questions to your exam board’s preferred style. There needs to be a clear gap (a line space) between the stem and options. Make use of diagrams and equations to avoid clunky explanations of practical apparatus and processes. Be deliberate in ordering the answers; numerical data should be in increasing order, dates chronological etc.
When listing elements, try to structure them logically – this is easier for students to process. Poor or unfamiliar formatting can impact accessibility of the question and hinder success.
| Example question | Explanation and improved question |
|---|---|
|
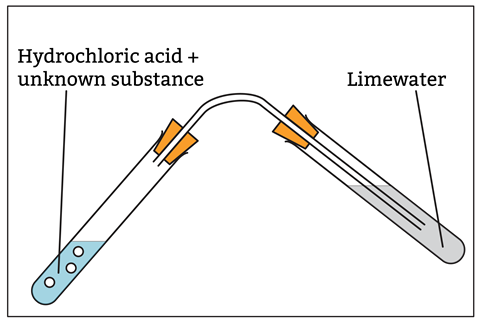
A student reacts an unknown substance with hydrochloric acid in a test tube. They collect the gas produced using a side-armed tube, bubbling it through limewater. The limewater turns cloudy. Which of the following have they identified with this test? A) Carbonate B) CO2 C) CO2- D) Carbon |
The stem is lengthy, with lots of information to process. The question also has two possible answers. While the question clearly requires students to recognise the test for carbonates in the unknown, the description is for the test for carbon dioxide gas. Instead, try: A student reacts an unknown substance with hydrochloric acid using the set-up shown below.
The limewater turns cloudy. State which anion they have identified with this test. A) CO32- B) CO2 C) CO D) C |
4. Use plausible alternatives
The keyword here is plausible. The optimum is three plausible alternatives, or distractors, which should be comparable in length and format to the correct answer.
The correct choice should require the use of specific cognitive processes; this avoids students selecting the right answer for the wrong reason, especially in calculation questions. Distractors should be representative of common misconceptions. Avoid using joke answers – these require no skill to rule out. Using all of the above or none of the above can make the cognitive demand too high, and you should use them with caution.
5. Take time to review
In his article, Gerry Everding notes that failure can strengthen the memory of misinformation, so it is vital that you review the test thoroughly and promptly with your students to avoid them absorbing the wrong information. I like to code each incorrect answer using misconception busters, such as these at post-16 for Electrochemical cells and Atom economy, percentage yield and green chemistry, enabling students to identify the next steps for moving their learning forward.
In his article, Gerry Everding notes that failure can strengthen the memory of misinformation, so it is vital that you review the test thoroughly and promptly with your students to avoid them absorbing the wrong information (bit.ly/40ouf95). I like to code each incorrect answer using misconception busters, such as at post-16 for ’Electrochemical cells’ (rsc.li/3GmG8Wc) and ’Atom economy, percentage yield and green chemistry’ (rsc.li/3TRXLA2), enabling students to identify the next steps for moving their learning forward.
6. Up the challenge
MCQs need to be challenging, but it is important to remember that we’re not trying to trap students. When choosing questions try to stick within students’ zone of knowledge. If you want to make the test harder, think about flipping the question or using assertion-reasoning to add complexity to the information presented.
You could try new visuals or less familiar experimental set-ups. Or give incomplete scenarios such as missing equipment labels or require students to identify mistakes.
| Example question | Explanation and improved question |
|---|---|
|
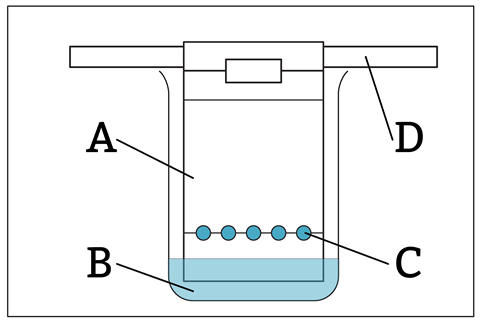
In chromatography, what is the stationary phase? A) The solvent B) The sample spots C) The beaker D) The chromatography paper |
This question involves simple recall, likely to be a regurgitation of students’ notes. Instead, try having diagrams which students need to interpret: A student uses chromatography to identify an unknown dye. They set up the apparatus shown below.
Which label shows the stationary phase? A B C D |
7. Make use of AI
AI can be very useful when creating MCQs. Try this prompt, editing the text in the brackets:
‘(Exam board & level) chemistry students are studying (topic and specification reference points). Produce (number) multiple choice questions, each with four possible answers, to test their understanding. The questions should be challenging, requiring students to (learning objectives). Give the correct answers and explain why the incorrect answers are wrong.’
More on AI
Find ideas to upskill learners to use AI, how to harness the tech for lesson planning and more on our Using AI in the chemistry classroom webpage.
It isn’t easy to create effective multiple choice questions, but with careful planning, you can use them to develop deeper understanding in your learners, leading to better long-term retention.
It isn’t easy to create effective multiple choice questions, but with careful planning, you can use them to instil a deeper understanding in your learners, leading to better long-term retention.
Louise Glynn










No comments yet