Use these approaches to ensure your 14–16 students are positive they understand ions
The relationship between atomic and ionic structure is a fundamental concept that can facilitate or hinder learners’ understanding in later topics. Properties of matter, ionic formulas, electrolysis and chemical cells all depend on secure knowledge of what an ion is and how it differs from a neutral atom.
1. Secure the basics
It’s always a good idea to recap what students learned at 11–14, but for this topic it is essential. Unless learners can confidently recall the charges of protons and electrons and understand why an atom has no overall charge, then introducing ion formation is unlikely to be successful. Going over the basics of atomic structure will make your lessons run more smoothly.
2. Use tools to help stuck students
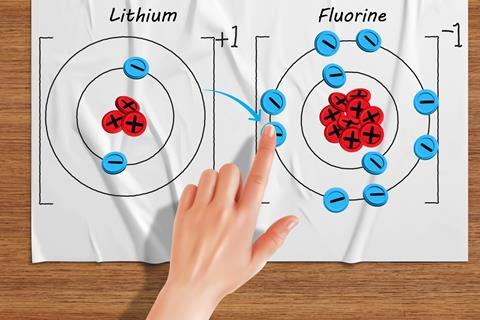
Some learners get a mental block and get stuck thinking that, for example, subtracting an electron means an atom becomes negative. Manipulatives are hands-on tools that are popular in mathematics education to make abstract concepts tangible and visible. Counters with plus and minus signs on them make good models for protons and electrons. Stick the protons down onto laminated atoms so learners can’t move them. Don’t stick the electrons so learners can exchange them between atoms. Physically counting the positive and negative counters can help students break down their misconceptions and secure the correct knowledge.
3. Model and practise language
Written explanations of ion formation often prove difficult for learners who write statements like ‘lithium gives one electron to fluorine’. The statement given is not correct; it suggests that lithium the substance is donating one electron, which of course it isn’t. Different exam authorities demand different exactitudes of language, so make sure you are familiar with how strict your own qualification is. Many mark schemes require students to be totally correct and write that the lithium atom loses one electron (from its outer shell) and donates it to (the outer shell of) the fluorine atom.
4. Diagnose misconceptions
When we assess students with quick-to-mark questions, like the numbers of protons and electrons in an ion, it is tempting to simply put a cross next to a wrong answer and move on. However, once you’ve finished marking, it is useful to revisit those questions to check if there is an underlying problem. Of course, sometimes students do just guess, but if you consistently see negative ions with the correct number of electrons subtracted rather than added then this may indicate a misconception you need to challenge. You could even add in a fun game to help develop fluency.
When we assess students with quick-to-mark questions, like the numbers of protons and electrons in an ion, it is tempting to simply put a cross next to a wrong answer and move on. However, once you’ve finished marking, it is useful to revisit those questions to check if there is an underlying problem. Of course, sometimes students do just guess, but if you consistently see negative ions with the correct number of electrons subtracted rather than added then this may indicate a misconception you need to challenge. You could even add in a fun game to help develop fluency (rsc.li/43shBYN).
5. Embrace anonymous ions
Students often work with just the first 20 elements in the periodic table, so they end up simply remembering common ions instead of deducing ionic charges. This is not necessarily a bad thing; we would love it if all learners could remember magnesium forms 2+ ions and hence magnesium chloride has the formula MgCl2. Yet, by removing familiar elements, we can encourage students to engage with the question more deeply. It also provides more practice than asking the same examples repeatedly. By using basic principles students can predict the charge of ions of unfamiliar elements, like V/V4+ or Fe/Fe3+. You can make questions even more challenging by using anonymous ions, such as M for a generic metal ion and X for a generic negative ion.
6. Use the periodic table as a cheat sheet
When students just need the ionic charge of a main group metal, the periodic table is the ultimate cheat sheet. Get learners to annotate their own copies with the ionic charges for elements in groups 1–3 and 5–7 and discuss their reasoning.
When students just need the ionic charge of a main group metal, the periodic table is the ultimate cheat sheet (rsc.li/3FSQXOU). Get learners to annotate their own copies with the ionic charges for elements in groups 1–3 and 5–7 and discuss their reasoning.
Use these tips to expand your toolkit of techniques in the classroom. Ensure you secure this key concept with your students to help them to embed their knowledge and succeed in more advanced topics later.
Kristy Turner















No comments yet