How researchers in Russia have found there’s still new discoveries on your dining room table
Download this
Download this starter slide for the topic of ionic structures with links to giant covalent structures for ages 14–16.
Download this
Use the starter slide with your class: rsc.li/XXX
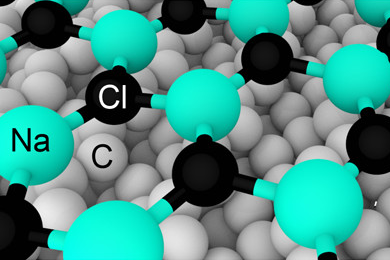
2D materials, which are made up of a single or a few atomic layers, have properties and structures that are often very different to the bulk compound. While some, like graphene, have been studied for decades, there are still many waiting to be discovered.
Table salt is one of the best known and simplest ionic compounds. It usually has a cubic crystal lattice structure. Yet when deposited as an ultrathin sheet on a diamond surface it forms a hexagonal structure. The discovery was made with the help of an algorithm, which the researchers used to predict crystal structures of sodium chloride on different metal and diamond surfaces. Salt and diamond showed a strong chemical interaction – which results in unusual structures.
Electron diffraction and X-ray analysis of samples confirmed that salt-on-diamond does contain hexagonal sodium chloride. As the thickness of the salt layer increases beyond 6 nm, the hexagonal structure reverts to the cubic one.
Use the starter slide with your class to help them understand different types of structures.
Read the full story in Chemistry World.
Downloads
Starter slide Hexagonal salt
Presentation | PowerPoint, Size 0.14 mbStarter slide Hexagonal salt
Presentation | PDF, Size 71.72 kb










No comments yet