Unusual isotopes of elements offer insight into the forces that hold atomic nuclei together
-

Download this
Use this story and the accompanying summary slide for a real-world context when studying atomic structure and isotopes with your 14–16 learners.
Download the story as MS Word or PDF and the summary slide as MS PowerPoint or PDF.
Researchers have created oxygen-28, the heaviest oxygen isotope ever, in a particle accelerator in Japan. Their experiments suggest that this isotope is not as stable as previously predicted.
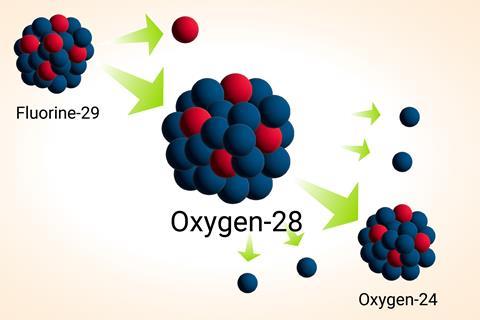
Yosuke Kondo, from the Tokyo Institute of Technology, led the large international team that produced oxygen-28 in the particle accelerator at the Riken Nishina Center. The researchers made the isotope by bombarding a beryllium target with calcium-48, an extremely rare natural isotope of calcium. The collisions caused some of the calcium-48 nuclei to fall apart, producing fluorine-29 nuclei. The fluorine-29 nuclei have the same number of neutrons as oxygen-28.
The scientists then fired the fluorine-29 nuclei at a liquid hydrogen target. This process knocked a proton off some of the fluorine-29 nuclei to produce oxygen-28 nuclei.
A fleeting glimpse
By studying isotopes with unusual ratios of neutrons to protons, researchers can gain insight into the structure of atomic nuclei and the forces that hold them together. Of particular interest are nuclei with ‘magic numbers’ of protons or neutrons, which are unusually stable compared to other isotopes of the same element.

Researchers had previously predicted that oxygen-28 nuclei would be ‘doubly magic’ because they have both a magic number of neutrons (20) and a magic number of protons (eight). As a result, they should be even more stable than nuclei with just a single magic number. However, Yosuke’s team’s experiments revealed that oxygen-28 nuclei only last for a fleeting moment. Within a fraction of a second, they rapidly lose four neutrons to form oxygen-24 nuclei.
This discovery exposes gaps in our understanding of what makes nuclei doubly magic and highlights that we need more fundamental research to better understand how neutrons and protons are held together in atomic nuclei.
This article is adapted from Jamie Durrani’s in Chemistry World.
Nina Notman
Reference
Y Kondo et al, Nature, 2023, DOI: 10.1038/s41586-023-06352-6
Download this
Summary slide with questions and the article for context when teaching 14–16 classes on atomic structure and isotopes: rsc.li/3Svu606
Downloads
EiC summary slide Heaviest oxygen isotope
Presentation | PDF, Size 0.21 mbEiC summary slide Heaviest oxygen isotope
Presentation | PowerPoint, Size 1.03 mbEiC science research story Heaviest oxygen isotope
Handout | PDF, Size 0.17 mbEiC science research story Heaviest oxygen isotope
Handout | Word, Size 0.5 mb















No comments yet