Studying molecular processes in real time
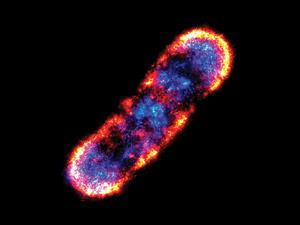
The 2014 chemistry Nobel prize has been given to three pioneers of biomedical imaging, whose work has enabled nanoscale features within cells to be captured in exquisite detail. Eric Betzig of Howard Hughes Medical Institute, US, Stefan Hell of the Max Planck Institute for Biophysical Chemistry, Germany, and W E Moerner of Stanford University, US, will share the prize for ‘the development of super-resolved fluorescence microscopy’.
The techniques they developed enabled extremely high resolution images to be produced using optical microscopy. Their work circumvented the problem of the ‘diffraction limit’ – the inability of light microscopy to distinguish between structures smaller than half the wavelength of visible light, or about 200 nm. This advance allowed nanoscale structures – including individual molecules – to be visualised within cells while they are still alive, something that isn’t possible with techniques such as electron microscopy.
‘Most of the processes in chemistry and biochemistry take place at length scales that are much smaller [than the wavelength of light],’ said Sven Lidin, of the chemistry Nobel prize committee, speaking at the announcement. ‘The work of the laureates has made it possible to study molecular processes in real time.’
In 2000, Stefan’s group at the Max Planck Institute developed Stimulated Emission Depletion (STED) to produce much higher quality light microscopy images. STED uses one laser to excite fluorescent molecules that have been introduced into a sample to help visualise it. A second laser is then used to quench the fluorescence of all but a tiny nanometre-sized area at the centre of the two lasers. By scanning the sample with these lasers, a single nanometre-wide strip at a time, a much higher resolution picture of structures like cell’s organelles can be built up, as the light from the other fluorescent molecules that would create a fuzzier picture is cancelled out.
Single molecule snapshots
Separately, Eric and Moerner – both working in industry at the time – laid the foundation for another technique called single fluorophore microscopy by showing that it was possible to detect the light emitted by a single fluorescent molecule. Working with this knowledge they showed it was possible to build up a high resolution image, snapshot by snapshot, by introducing tiny amounts of fluorescent proteins into a sample. They created their high resolution image by exciting just a few fluorescent proteins in a sample at a time using a weak light pulse. As the fluorescent proteins were few and far between in the sample, those that glowed were generally more than 200 nm apart – the diffraction limit for optical microscopy. When the excited fluorescent proteins had ‘burnt out’ they repeated the excitation process again and again, taking lots of snapshots of the fluorescent proteins spread throughout the sample. These images could then be overlaid to build up a high resolution picture of the sample, once again circumventing the limit imposed on optical microscopy by light’s wavelength.
In the years since, these imaging tools have enabled molecular biologists to track the movement of signalling molecules or disease markers within individual cells, for example mapping the molecular changes within an individual nerve cell during learning.
Jennifer Lippincott-Schwartz, a cell biologist at the US National Institutes of Health, says these techniques are ‘revolutionising our concepts of some of the most fundamental concepts of living cells, which until now were hidden’. Her group use super-resolution imaging to investigate how disease-causing bacteria and viruses interact with cells. ‘The techniques could even be used in identifying unique features of the Ebola virus to help find new ways to abolish it,’ she suggests.
Overcoming challenges
Speaking by telephone at the announcement, Stefan told reporters he was ‘totally surprised’ to have received the award. Later, he talked about how his ideas were challenged during the early stages of his research. ‘Because I knew that this idea was fundamentally correct, I held on to it and did not allow myself to be misled when people told me it wouldn’t work,’ he said. ‘There were certain points that had to be overcome and solved, but they were not basic, which is why I believed in it,’ he added. ‘There is a big difference between having a technical problem and having a basic problem – the basic ones will probably remain; technical problems can be overcome.’
Steven Lee from the University of Cambridge, UK, who was a postdoctoral researcher in Moerner’s lab at Stanford, thinks the news is ‘outstanding’. ‘These exciting discoveries have emerged through painstaking years of research by physical scientists trying to better understand how light interacts with matter at a fundamental level,’ he says. ‘I was lucky enough to work with W E Moerner – his curious and passionate approach to science is an inspiration to us all.’









No comments yet