All Spectroscopy articles
-
 News
NewsImprove your learners’ NMR interpretation skills
Encourage students to determine and draw the structures of simple organic molecules with this free, online resource
-
 Job profile
Job profileScientist, food and pharmaceuticals
Taryn uses scientific instruments to test our food and medicines and make sure they are safe
-
 CPD article
CPD articleTeaching spectroscopic techniques at post-16
Use these ideas and resources to help your students master spectroscopy
-
 Resource
ResourceAnalytical chemistry in Ireland
Worksheets, project-based learning tasks and contextualisation stories to develop a core understanding of analytical chemistry
-
 CPD article
CPD articleHow to teach structure determination post-16
Students find this process puzzling. Help them solve it with these ideas and tips
-
 Resource
ResourceStructure determination | 16–18 years
Test learners’ ability to identify the spectra of given compounds and apply their understanding of infrared and NMR spectroscopy with this worksheet
-
 Ideas
IdeasTeach justice and cooperation through chemistry
Link forensic science and the Covid-19 pandemic to your lessons on instrumental analysis, separation techniques, data interpretation and health with UN sustainable development goals 16 and 17
-
 Interview
Interview‘You don’t need to choose between the arts and science’
Meet Katherine Curran, a chemist applying her polymer know-how to the conservation of plastic museum objects
-
 Feature
FeaturePlastic conservation
Usually we want plastics to degrade, so what about when we don’t? How chemists are helping museums preserve plastics
-
 Ideas
IdeasBack to basics with spectrophotometry
How to demonstrate the Beer–Lambert law using your smartphone as a light meter
-
 Resource
ResourceSmartphone spectroscopy: Beer–Lambert law
Use your smartphone to measure changes in concentration across different concentrations of squash at home or in the classroom. Use your results to predict the concentration of an unknown dilution of squash.
-
 Feature
FeatureThe science of smartwatches
How wearable tech uses chemistry to help monitor your health – with spectroscopy, battery technology and smart materials
-

-
 Feature
FeatureHow chemists are stopping paint drip
Analytical chemistry is playing an important role in discovering why some 20th century paintings have started to liquefy
-
 Ideas
IdeasLearning at home for 16–18
Use these curriculum-relevant resources and activities remotely with your 16–18 classes
-

-

-
 Resource
ResourceStructure determination 16–18
Practice interpreting 1H and 13C NMR, mass spectra, and thin layer chromatograms with these Starter for ten questions.
-
 Resource
ResourceAnalysis 16–18
Practice using analysis skills with these mass spectrometry and infra-red spectroscopy Starter for ten questions.
-
 Resource
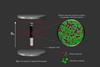
ResourceNuclear magnetic resonance (NMR) spectroscopy: Hydrogen
Numclear magnetic resonance (NMR) is particularly useful in the identification of the positions of hydrogen atoms (1H) in molecules. This is an invaluable technique in the identification of organic compounds and commonly used in analytical laboratories











