Fifty years ago steroid abuse among sportsmen and women was a serious problem. Today, thanks to the skills of analytical chemists, the sporting cheats rarely win
In Short
- Testosterone and its derivatives were exploited by athletes to boost their strength and stamina from the early 1950s to the 1990s
- Today's chemists, equipped with high-performance analytical techniques, are one step ahead of the sporting cheats
As a family of molecules, steroids have few equals in terms of importance in their function in the human body. Their diverse properties have also made them a target for misuse, notably as performance-enhancing drugs in competitive sports.

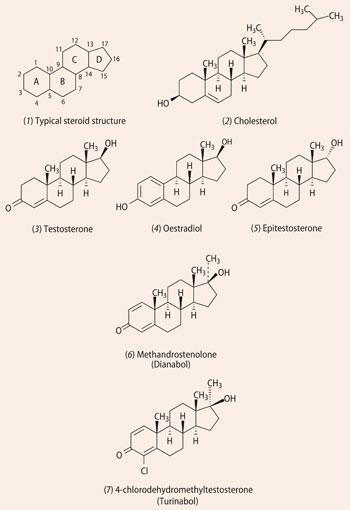
Steroids are molecules that contain four rings fused together in a cyclopentaphenanthracene system: three of the rings are six-membered, one is five-membered, see structure (1). Among this family of molecules, cholesterol (2) performs several vital roles in the body - it is involved in the production of bile acids, it is the precursor of sex hormones (also steroids), and it is needed to make vitamin D, among many other functions.
Here we focus on the sex hormones and their derivatives, and how they have been misused by sportsmen and women to give them the advantage in world class competitions.
The sex hormones
Both sexes carry some of each of the principal sex hormones, testosterone (3) and oestrogens, but there are generally significantly higher levels of testosterone in men than in women, and conversely more oestrogens such as oestradiol (4) in women.
Testosterone (3) is synthesised in the male testes from cholesterol. As an anabolic steroid it promotes the development of muscle mass and strength. It is oxidised to androstenedione, which is excreted in the urine, and has an inactive epimer (isomer differing in only one stereogenic centre, in this case C17) called epitestosterone (5).
Oestradiol (4), along with its oxidation product oestrone, and oestriol, are the main female sex hormones, responsible for the development of breasts and the regulation of the menstrual cycle. In contrast to testosterone, the female sex hormones feature a benzene ring as ring A in the steroid structure.
Early misuse of steroids
Weightlifters began to use steroids after World War II. Suspicions that the Soviet competitors of the early 1950s were using testosterone as a way of building muscle led American weightlifters to try it.

In 1958, methandrostenolone, marketed as Dianabol (6), was released as a drug that gave anabolic effects without androgenic side-effects, ie secondary male characteristics such as beard growth and deepening of the voice. The presence of a methyl group in the 17α position on ring D makes the molecule a tertiary alcohol, which does not easily break down (metabolise) in the liver, so it could be taken orally. (In contrast testosterone was given intravenously by injection.) However, this also makes the molecule hepatotoxic, ie over time it causes liver damage. Dianabol was widely used by field athletes and body builders, including Arnold Schwarzenegger, prior to it being banned in the 1990s.
Around this time the International Olympic Commission(IOC) introduced drug testing, but its main focus were substances like amphetamines rather than steroids. Routine testing for steroids was initiated at the 1976 Olympic Games in Montreal, Canada.
By the mid-1960s chloro-substituted Dianabol was available as Turinabol (7). This 4-chlorinated product could also be given orally and is metabolised in the body relatively quickly, giving a low risk of detection in early steroid screens. In a controlled programme run under the scrutiny of East German secret police, the Stasi, many athletes and swimmers were given Turinabol under the guise of vitamins. Additionally, some competitors were given testosterone injections.
The effects upon performance were most apparent in the women's swimming events. At the 1976 Montreal Olympics, East German women took 11 of the 14 gold medals and though some teenage girls also developed masculine traits, such as body hair and deep voices, this didn't seem to concern them. More serious health problems became apparent some years later. Detlef Gerstenberg, who threw the hammer in the 1980 Olympics, was hospitalised at the age of 35 with extensive liver damage and died following post-operative complications in January 1993.
After the fall of the Berlin Wall, the Stasi files came under investigation.1 As a result of this work, the two leaders of the programme, Manfred Ewald and Manfred Hoeppner, were brought to trial in 2000, and found guilty of inflicting bodily harm upon more than 100 young women athletes.
Detecting steroids
The usual method of detecting steroids relies upon identifying their metabolites. While some steroids are excreted unchanged, most undergo processes such as hydroxylation, oxidation or reduction, as well as conjugation as a glucuronide. These metabolites are excreted in the urine or faeces.
After extraction from the urine, any glucuronides have to be hydrolysed with a β-glucuronidase enzyme, and the steroids converted into trimethylsilyl derivatives, which are volatile compounds and can be analysed by using GC-MS.2
In the early 1980s, Professor Manfred Donike, head of the IOC-approved testing laboratory in Cologne, Germany, detected testosterone abuse by measuring the relative amounts of testosterone (3) and its inactive epimer, epitestosterone (5), in urine samples.
The natural ratio of testosterone (T) to epitestosterone (E) in adults is 1:1. Although a ratio of T:E > 2:1 would imply that testosterone had been introduced into the body, in 1982 the IOC set a value of 6:1 as one that would result in immediate disqualification.
This test resulted in many athletes being disqualified, but did not stop others trying to find ways of avoiding detection. The East Germans, for example, found that esters such as testosterone propionate were metabolised rapidly by the body, so they would give their athletes this compound in place of other steroids a few weeks before a competition and then stop all steroid use about four days before the competition so that the athletes' T:E ratios fell below 6:1. Others would take testosterone-epitestosterone mixtures, which also kept the T:E ratio below 6:1.
Among the athletes convicted on the basis of a T:E ratio greater than 6:1 was the cyclist Floyd Landis, who was stripped of the 2006 Tour de France title after drug testing revealed a T/E ratio of 11:1 in his urine sample.
Nowadays, if the T:E ratio is above 4:1, laboratories also run a carbon isotope ratio test (CIR).3 This determines the 13C/12C ratio in the testosterone sample. Testosterone made naturally in the body from cholesterol will have a different 13C/12C signature, as revealed by mass spectrometry, from synthetic testosterone which is made from phytosterol percursors, typically derived from wild yams or soy. The synthetic testosterone has a lower 13C/12C ratio, so the test can confirm if someone has taken testosterone.

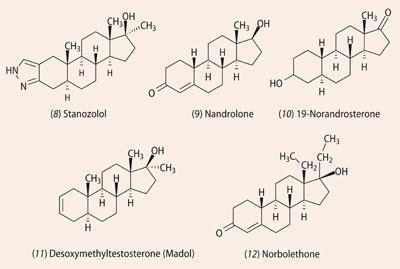
At the 1988 Olympics in Seoul, South Korea, the Canadian 100-m sprinter, Ben Johnson, was stripped of his gold medal for testing positive for stanozolol. Stanozolol (8) is a synthetic anabolic steroid derived from testosterone and was widely used by athletes because it develops muscle without weight gain and so gives 'lean' body mass. This steroid is metabolised relatively quickly mainly to hydroxylated steroids, which can be converted into volatile trimethylsilyl derivatives and detected by GC-MS.4
More recent times
During the 1990s nandrolone (9) became a favourite anabolic steroid for bodybuilders who wanted to build muscle. This steroid is present naturally in minute (ng) quantities and is detected (in the urine) as its breakdown product, 19-norandrosterone (10).
Several athletes, including the British sprinter Linford Christie have tested positive for nandrolone, as well as footballers Christophe Dugarry and Edgar Davids, the Czech tennis star Petr Korda and Pakistani Test cricketer Shoaib Akhtar. In some cases the levels of norandrosterone were up to 50 times the maximum 'natural' amount.
In a surprising development, scientists at Aberdeen University found that a combination of allowed dietary supplements, such as creatine, together with hard cardiovascular exercise can lead to norandrosterone levels over five times the legal maximum. It is unlikely, however, that this could account for all the cases.
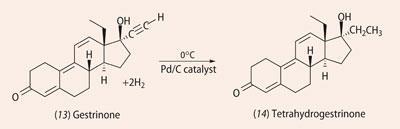
As chemists got better as identifying steroids, people sought new ways around this. Patrick Arnold, an American chemistry graduate and a bodybuilder, studied the steroid literature, focusing on molecules that had been reported in the past but which were never commercialised. He became involved with the Bay Area Laboratory Cooperative (BALCO), producing 'designer' anabolic steroids that had never come to market and were not covered by routine drug testing. The three main molecules were desoxymethyltestosterone5 ( 11, Madol); norbolethone6 (12), and tetrahydrogestrinone7 (14, THG). Madol and norbolethone had been synthesised in the 1960s and investigated, but neither had been marketed. THG had never been made before
BALCO provided undetectable performance-enhancing supplements to many top athletes. Then on 5 June 2003 an 'anonymous' track coach contacted a US Anti-Doping Agency (USADA) official, naming Victor Conte, owner of BALCO, as a person supplying top athletes with drugs, and sent the USADA a used syringe that had been dumped in a trash can at a meeting. USADA sent the syringe to Don Catlin, head of the UCLA (University of California, Los Angeles) Olympic Analytical Laboratory, who eventually identified the chemical as tetrahydrogestrinone. There followed a police raid on BALCO, which uncovered lists of 'customers', and led to the imprisonment of Conte and Arnold.
Winning - but at what cost?
In the past, many steroid users in the world of sport were unwitting victims. The 1980 Olympic triple gold medallist Rica Reinisch retired from swimming aged 16 because of ovarian cysts. She suffered two miscarriages and has a permanent heart problem. Commenting on her plight, she said: 'The worst thing is they took away from me the opportunity to ever know if I could have won the gold medals without the steroids. That's the greatest betrayal of all'.

Nowadays, scientists are much better equipped to detect the use of steroids in sport, yet some individuals go on trying to beat the system.
Dr Simon Cotton recently retired from teaching chemistry at Uppingham School and can be contacted by e-mail at sac@uppingham.co.uk.
Further Reading
- Steven Ungerleider, Faust's gold: inside the East German doping machine. New York: Thomas Dunne Books, 2001.
- M. Fainaru-Wada and L. Williams, Game of shadows: Barry Bonds, Balco, and the steroids scandal that rocked professional sports . New York: Gotham Books, 2006.
- J. McCloskey and J. E. Bailes, When winning costs too much: steroids, supplements and scandal in today's sports world. Lanham, Maryland: Taylor Trade, Lanham, 2005.
- David Walsh, From Lance to Landis: inside the American doping controversy at the Tour de France . New York: Ballantine Books, 2007.
- A. T. Kicman, Br. J. Pharmacol, 2008, 154, 502; T. Todd, J. Sports Hist., 1987, 14, 87.
How Don Catlin's team cracked THG
On 13 June 2003, Don Catlin received a methanolic solution of an unknown steroid, recovered from a hypodermic syringe. He ran standard GC-MS tests on the solution, and synthesised several derivatives. Attempts to identify the steroid failed because the mass spectrum contained a large number of unidentifiable peaks. The only compound that they could identify at this stage was a small amount of another anabolic steroid, norbolethone, evidently present as an impurity. Catlin suspected that the 'unknown' shared a common carbon skeleton with norbolethone. However, they noted a peak in its mass spectrum with m/z = 312, and thought this was the molecular ion. Accurate mass measurement gave 312.2080, from which they deduced the compound had the molecular formula C21 H28 O2.

When they compared the mass spectrum of the unknown with other steroids, it became clear that it shared features with gestrinone and trenbolone. All three compounds had the same fragments with m/z values at 211 and below present, so Catlin deduced that they contained the same A, B and C rings.
Furthermore, when the MS of the unknown was compared with gestrinone, the fragments with m/z above 240 occur 4 Da higher in the unknown, suggesting that it was gestrinone with four additional hydrogen atoms. A possibility was that the terminyl alkyne group in gestrinone had been reduced to an ethyl group.
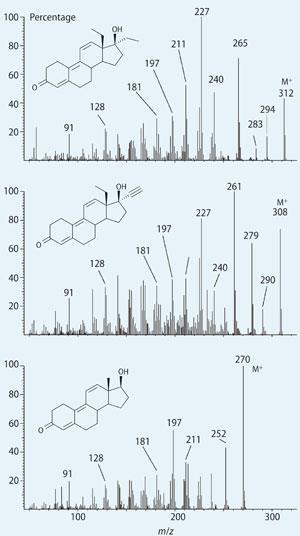
Having tentatively identified the unknown steroid as tetrahydrogestrinone, the team then prepared an authentic sample of THG by catalytic hydrogenation of gestrinone. This required careful control of conditions (0ºC) to prevent hydrogenation of C=C double bonds (see equation).The retention time and mass spectra of the synthetic THG matched the unknown material exactly.
To study the metabolism of THG in mammals, the team gave intravenous doses of THG to a baboon, and collected urine samples from the animal over several days. Detectable amounts of THG were found in urine for many hours after administration.
THG was thus directly detectable in urine samples, though it defies detection by the standard procedure involving derivatisation into the Me3 Si derivatives.
References
- W. W. Francke and B. Berendonk, Clin. Chem ., 1997, 43, 1262.
- W. Schauzer and M. Donike, Anal. Chim. Acta, 1993, 275, 23.
- R. Aguilera, C. K. Hatton and D. H. Catlin, Clin. Chem., 2002, 48, 629.
- R. Masse, C. Ayotte, H. Bi and R. Dugal, J. Chromatog., 1989, 487, 17; S. Poelmans et al, Anal. Chim. Acta., 2002, 473, 39.
- M. H. Sekera et al, Rapid Commun. Mass Spec., 2005, 19, 781.
- D. H. Catlin, B. D. Ahrens and Y. Kucherova, Rapid Commun. Mass Spec., 2002, 16, 1273.
- D. H. Catlin et al, Rapid Commun. Mass Spec., 2004, 18, 1245.









No comments yet