Stilton, camembert, limburger and cheddar - why, and how, does cheese come in such a variety of smells and tastes?
- Cheese-making results in a complicated interlocking series of reactions, leading to a huge range of different aromas and tastes in ripened cheeses
Charles de Gaulle's famous 1962 remark 'How can anyone govern a nation that has 246 different kinds of cheese?' is a reminder of the sheer variety of gustatory experience provided by cheese, which has been made for over 4000 years. The discovery of cheese provided the ultimate long-life milk, a foodstuff containing a lot of protein and fat, a source of essential amino acids as well as vitamins and minerals (eg calcium). So why is stilton so different to cheddar and to camembert, and why does limburger smell of sweaty feet?

Making the cheese
Most cheese is made from cow's milk, which is usually pasteurised by brief heating (to 70°C), killing any undesirable bacteria, then cooled. Rennet and "starter" bacteria (usually from the Streptococci and Lactobacilli families) are added and the mixture digested for an hour or so at 30-40°C; the bacteria ferment lactose to form lactic acid, reducing the pH to a value circa 4.6 where enzymes such as chymosin (rennin) can coagulate the casein, the predominant protein in the milk, forming curds. The starter bacteria also fulfil other roles, including metabolising citric acid and helping to break down the protein. The warm curds are allowed to set for an hour or two before the liquid whey is separated from the curds by cutting the curds into small pieces.

Cottage cheese is made from drained cheese curds, it is not aged before eating, so it tastes much blander than ordinary cheese, as there has been little time for ripening to generate flavour volatiles. In hard cheeses like parmesan, cheddar and gouda, the starter bacteria are also the ripening agents, whilst blue cheeses have the blue-green Penicillium roqueforti mould added, and are then pierced to allow oxygen access to the bacteria. This piercing speeds up the ripening. Camembert cheese is ripened from the outside inwards thanks to a white coating of Penicillium camemberti mould, and washed-rind cheeses including limburger and brick are surface-ripened by Brevibacterium linens bacteria on the surface. In the case of harder cheeses (eg St Nectaire, cheddar, cantal, emmental, parmesan), the curds are warmed to release more whey, before being salted and pressed.
Cheeses are stored to allow them to ripen, usually at 5-10°C. Different types of cheese require different ripening times. Soft cheeses contain more water and therefore favour bacterial growth and ripen faster. So whilst hard cheeses such as parmesan may ripen for a year and more, camembert will be ready in three or four weeks.
Flavour molecules
The molecules we smell come from breakdown of the three types of chemical in the milk - the protein casein; lipids in milk fat; and lactose (Table 1). The volatile compounds formed depend upon the particular microbes acting as ripening agents as well as other factors, such as the type of milk used. Goat and sheep milk are richer in lipids containing short-chain fatty acids, leading to smaller, more volatile, odorant molecules.
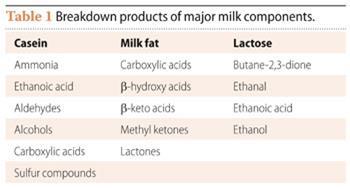
Simplified versions of the three pathways are shown in Scheme 1, Scheme 2 and Scheme 4.
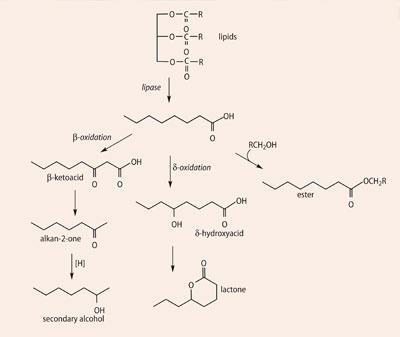
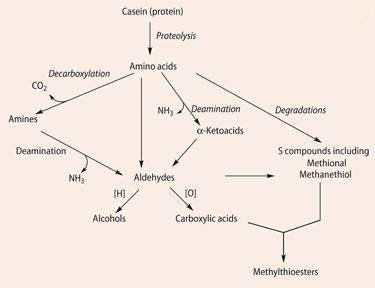
Lipolysis (Scheme 1) yields carboxylic acids, the source of a range of smelly molecules. By themselves, carboxylic acids would not give a cheese a wholesome aroma, so the presence of other molecules is necessary. The fatty acids are the source of the methyl ketones that give 'blue cheese' notes, and also react with alcohols, especially ethanol, to afford a range of flavoursome esters. Proteolysis (Scheme 2) breaks down proteins like casein - first into peptides and then into amino acids. These contribute taste to cheese but more importantly undergo a wide range of transformations - decarboxylation, deamination, oxidation and reduction - again affording a whole range of short-chain volatiles. Examples of transformations possible with valine are shown in (Scheme 3). Breakdown of lactate and citrate (Scheme 4) produces important molecules like diacetyl (a 'buttery' taste), ethanal and ethanol.
Any ripened cheese contains a mixture of many volatile molecules, and the overall smell is due to that blend, though one particular type of odorant can occasionally be dominant, as in blue cheeses. In some cases (St. Nectaire, cantal, ragusano), cheeses have been found to contain terpene molecules derived from plants, consumed by cows on mountain pastures, which enrich the flavour.
Here are four types of cheese with distinctive odours, illustrating how the cheese smells arise.
Blue cheeses
Stilton, roquefort, gorgonzola and other blue cheeses have a distinctive aroma caused by certain methyl ketones (alkan-2-ones).1 Heptan-2-one, and to a large degree nonan-2-one, are most often linked with blue cheese smell; pentan-2-one is usually described as fruity.
The alkanones originate in carboxylic acids, produced by the lipolysis of triglycerides (Scheme 1); the acids are then catabolised by the P. roqueforti, being oxidised to σ-hydroxyacids and to β-ketoacids, before decarboxylation to ketones which have one carbon atom fewer than the initial acid. Thus the even-carbon acids generally found in lipids form odd-carbon ketones. Short-chain C5 to C10 acids are rare in lipids, and repeated β-oxidation of the acids may be needed to generate these alkan-2-ones.
Heptan-2-one is the most abundant ketone in blue stilton, with significant amounts of butan-2-one and pentan-2-one;2 a comparison of roquefort with bleu d'auvergne and bleu des causses found heptan-2-one and nonan-2-one to be most abundant in the first two but pentan-2-one to be the most abundant in bleu des causses.3 Other compounds, including alcohols and esters, round out the flavours; thus in gorgonzola, heptan-2-one and nonan-2-one are key impact molecules, but 1-octen-3-ol, 2-heptanol, ethyl hexanoate and methylanisole are also important odorants.4
Camembert
Lactate metabolism is vital in surface mould-ripened cheeses such as camembert and brie. Initial growth of microorganisms such as Geotrichum candidum on the cheese is soon followed by a surface mould of P. camemberti . This breaks down lactic acid (itself a breakdown product of lactose) to carbon dioxide and water. Removing the acid causes the pH of the surface to increase from ca. 4.6 to 7.

At this pH, calcium phosphate is insoluble, so it precipitates on the surface, setting up a concentration gradient that draws calcium phosphate away from the centre, and removing it from binding the casein micelles together. This is one process responsible for the softening of the centre of camembert as it ripens.
Ripe camembert often smells of ammonia, produced by deamination of amino acids on the surface, but the odour of camembert is due to a number of compounds as several reactions are involved in its ripening, with 1-octen-3-ol, 1-octen-3-one, 3-methylbutanal, 2-undecanone, butane-2,3-dione, d-decalactone, butanoic acid, 3-methylbutanoic acid, methional, dimethylsulfide and methanethiol as key contributors to its impact.5 1-octen-3-ol and 1-octen-3-one provide a mushroom note and are produced by the action of lipoxygenases in P. camemberti upon linoleic acid. Butane-2,3-dione (metabolism of citrate (Scheme 4)) gives a buttery effect.
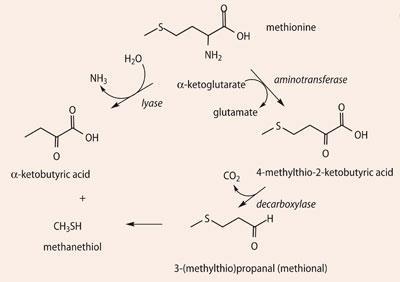
Thanks to Geotrichum candidum and P.camemberti, the sulfur-containing amino acid methionine undergoes catabolysis in various ways (Scheme 5), forming several sulfur compounds which have a marked influence on the aroma of traditional camembert, imparting a flavour of garlic and cabbage to matured cheeses. Methanethiol is an important odorant and a source of Me2 S, Me2 S2, Me2 S3 and other sulfur compounds. Methional is another important odorant found in many cheeses. Although by itself methional's boiled potato smell is not particularly pleasant, in combination with other volatiles it is just one note of the cheese aroma of camembert and cheddar. It is rare that one molecule carries the smell of a particular cheese, but S -methylthiopropionate, CH3 CH2 C=O(SCH3), does indeed smell like camembert. Found in camembert made from raw milk, it probably results from reaction of the appropriate acyl-CoA (propionyl-CoA) and CH3 SH.6
Limburger
Another quite distinctive aroma is that of limburger, a surface-ripened semi-soft cheese. In this cheese the drained and cut curds are rolled in salt and regularly brushed with a solution infused with B. linens. The brine prevents other bacteria from interfering and extensive lipolysis occurs, with enzymes degrading casein. This causes significant formation of several carboxylic acids including the volatile butanoic, 3-methylbutanoic and hexanoic acids, so that the surface rind develops the 'sweaty feet' aroma characteristic of limberger,7 which diffuses through the cheese.

The closely related Brevibacterium epidermidis is found on human skin, where it breaks down lipids, forming these same carboxylic acids responsible for foot odour.
Interestingly, these acids also make limberger cheese an attractant for female Anopheles gambiae mosquitoes, so this cheese can be used as bait to trap them (Dutch scientists Bart Knols and Ruurd de Jong shared the 2006 Ignobel prize in Biology for this work).8
Other aroma molecules that contribute to the smell of limberger include methionine-derived methanethiol and methylthioacetate. CH3 C(=O)SCH3.
Hard cheeses
Cheddar is the best-known cheese of this type in Britain, though there are many other familiar names throughout Europe, including cantal, parmesan and edam. In these cheeses, the ripening process is carried out evenly throughout the cheese by the starter bacteria, in a process occurring over many months. Various studies have identified over 100 different odorants in cheddar cheese, with ethanoic acid (sharp), butyric acid (sweaty, sweet), d -dodecalactone (coconut), methional (boiled potato), furaneol (caramel), homofuraneol (caramel) and butane-2,3-dione (buttery) believed to be important.
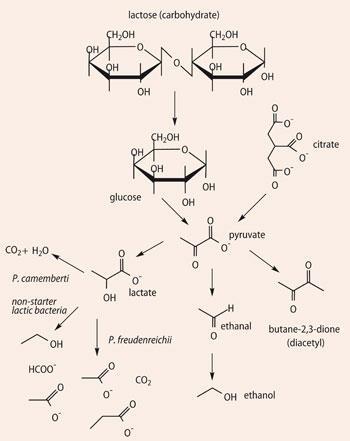
The acids are formed through lipolysis of casein, whilst the lactone is a result of internal esterification of a lipolysis-derived hydroxy acid (Scheme 1). Esters like ethyl butanoate and ethyl hexanoate result from reaction of the free acids with ethanol (a product of pyruvate metabolism). Butane-2,3-dione is a metabolite of pyruvate, itself derived from both lactose and citric acid (Scheme 4), and methional is formed by catabolism of the amino acid methionine (Scheme 5). No single molecule dominates the aroma, which is perceived to be due to a balanced blend of the components, with various notes identified - sour, salty, nutty, fatty acid, cowy, brothy, buttery and sweet.9, 10
Studies of other hard cheeses have identified similar lists of molecules, with ethanoic and butanoic acids, diacetyl and methional common to both cantal11 and parmesan.12
Variants on these hard cheeses are gruyère and emmental, cheeses which especially in French versions often contain 'eyes' or holes. After lactose fermentation, a secondary fermentation brought about by the bacterium Propionibacterium freudenreichii converts lactate into ethanoate, propanoate and also gaseous carbon dioxide (Scheme 3). The CO2 migrates through the cheese, accumulating and forming 'eyes' in the developing cheese.
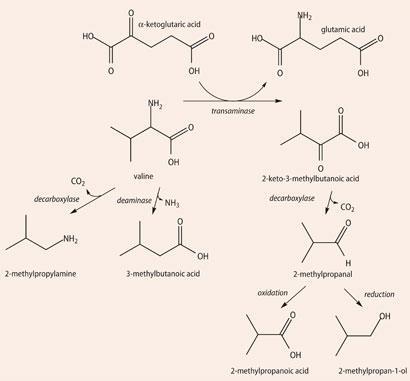
Gruyère odorants13 also include butanoic, 2- and 3-methylbutanoic and phenylethanoic acids, methional and 2- and 3-methylbutanal. 3-methylbutanal supplies malty and nutty notes to several cheeses and is formed in the cheese from the amino acid leucine by catabolysis. First the leucine undergoes transamination into a keto acid and then another enzyme catalyses the decarboxylation of the acid, forming the aldehyde.
Conclusion
Though flavour molecules are only derived from three types of starting material in cheese curds, cheese ripening involves a complicated interlocking series of reactions, leading to a huge range of different aromas and tastes in ripened cheeses.
Further Reading
- H. McGee, McGee on food and cooking: An encyclopedia of kitchen science, history and culture, London: Hodder, 2004, pp 51-67.
- P. M. G. Curioni and J. O. Bosset, Int. Dairy J., 2002, 12, 959 (key cheese odorants).
- Y. F. Collins, P. L. H. McSweeney and M. G. Wilkinson, Int. Dairy J., 2003, 13, 841 (lipolysis and free fatty acid catabolism in cheese).
References
- J. B. Lawlor et al, Int. Dairy J., 2003, 13, 481.
- K. Gkatzionis, R. S. T. Linforth and C. E. R. Dodd, Food Chemistry, 2009, 113, 506.
- A.Gallois and D. Langlois, Lait, 1990, 70, 89.
- L. Moio, P. Piombino and F. Addeo, J. Dairy Res., 2000,67, 273.
- J. Kubícková and W. Grosch, Int. Dairy J., 1998, 8, (a) 11; (b) 17.
- C. Berger et al., J. Agric. Food Chem., 1999, 47, 3274.
- T. H. Parliment, M. G. Kolor and D. J. Rizzo, J. Agric. Food Chem., 1982, 30, 1006.
- B. G. J. Knols et al., Bull. Entomol. Res., 1997, 87, 151.
- O. Suripaphan et al., J. Agric. Food Chem., 2001, 49, 1382.
- T. K. Singh, M. A. Drake and K.R. Cadwallader, Comp. Rev. Food Sci. Technol., 2003, 2, 139.
- A. Cornu et al., Int. Dairy J., 2009, 19, 588.
- M. Qian and G. A. Reineccius, J. Dairy Sci., 2003, 86, 770.
- M. Rychlik and J. O. Bosset, Int Dairy J., 2001, 11, 895.









1 Reader's comment