Get your students up and out of their seats or working with their hands to embed learning about challenging chemistry topics
Our role as teachers is to help students understand chemistry so they can explain what happens during a particular process or to cause a specific outcome. Some topics are difficult for students to grasp, perhaps because what’s happening can’t easily be observed, or perhaps because the concepts are challenging. Either way, using models can help students to make sense of what is going on.
To help students make links between a model and what it represents, we need to introduce and use key vocabulary associated with the concept in our explanations. With the process of polymerisation, for example, large numbers of identical molecules (monomers) join (bond) to produce long chains of repeating units (polymers). This can be modelled in several different ways. We can fold and cut to make long chains of paper dolls, where each doll represents the repeat unit of the polymer. Alternatively, students can model the process physically.
Our role as teachers is to help students understand chemistry so they can explain what happens during a particular process or to cause a specific outcome. Some topics are difficult for students to grasp, perhaps because they can’t easily observe what’s happening, or because the concepts are challenging. Using models can help students make sense of it all.
To help students make links between a model and what it represents, we need to introduce and use key vocabulary associated with the concept in our explanations. With the process of polymerisation, for example, large numbers of identical molecules (monomers) join (bond) to produce long chains of repeating units (polymers). This can be modelled in several different ways. We can fold and cut to make long chains of paper dolls, where each doll represents the repeat unit of the polymer. Alternatively, students can model the process physically.
Actively learning
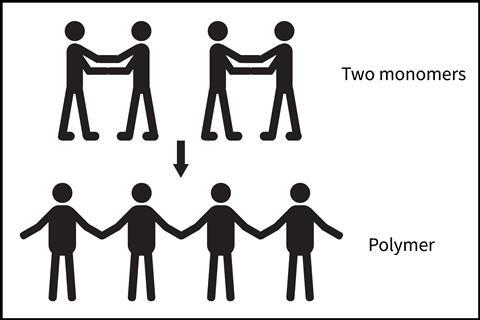
To physically model polymers, students stand in two pairs and hold each other’s wrists. Each pair represents a monomer, with the benefit of the two joined arms being like a double bond in an alkene. Each student then lets go of one of their partner’s wrists, so one arm is joined and the other is free. The two students in the centre (standing next to each other) each grasp the other’s free wrist – forming a four-person chain – with a free arm at each end for further repeat units to add onto.
As students progress through this process, describe it using key vocabulary. My student monomers represent atoms joined by a double bond; one bond is then broken so the repeat units can form the required new bonds to make an addition polymer. Breaking the process down into smaller steps helps students understand what’s happening at the sub-microscopic level – that bonds need to break before new ones are made.
Check understanding by questioning. For example:
- What does each monomer need for addition polymerisation to occur? A double bond.
- What does each pair of students in the chain represent? A repeat unit.
You could also discuss the limitations of the model – ie the students (and hence the repeat units) aren’t identical.
To physically model polymers, students stand in two pairs holding each other’s wrists. Each pair represents a monomer, with the benefit of the two joined arms being like a double bond in an alkene. Each student then lets go of one of their partner’s wrists, so one arm is joined and the other is free. The two students in the centre (standing next to each other) each grasp the other’s free wrist – forming a four-person chain – with a free arm at each end for further repeat units to add onto.
As students progress through this process, describe it using key vocabulary. My student monomers represent atoms joined by a double bond; one bond is then broken so the repeat units can form the required new bonds to make an addition polymer. Breaking it down into smaller steps helps students understand the sub-microscopic level – that bonds need to break before new ones are made.
Check understanding by questioning. For example, ask what each monomer needs for addition polymerisation (a double bond) or what each student pair in the chain represents (a repeat unit).
You could also discuss the limitations of the model – ie the students (and hence the repeat units) aren’t identical.
A learning tangle
I use another model to demonstrate fractional distillation with different lengths of strings. This involves cutting about 20 pieces of string to a variety of lengths, from just a few centimetres to around 30 centimetres. Place the strings in an opaque plastic beaker with the free ends hanging over its edge. Push the other ends into the beaker so the ends tangle together. Invite a few students to each loosely take hold of a string end and then try to remove it. The longer the string, the more tangled it will likely be, and all the harder to pull out (without dislodging all the strings).
Explain that the strings of different lengths represent different fractions of oil with different chain lengths. The longer chain molecules have more points of contact and so require more energy to separate them from the mixture, which means the temperature will be higher. You can also tie knots in the pieces of string to represent carbon atoms. So, the shortest string would have one knot and the longest string would have the most knots (as evenly spaced as is possible). This helps the strings tangle together, becoming more difficult to separate.
Then ask students to repeat the exercise as well as explain what’s happening. You could perhaps give some key vocabulary or ask questions to scaffold. You can also use Molymods or similar to build models of alkanes with different lengths which can be muddled together in a Gratnells tray. Be clear that we are not breaking bonds within the molecules but rather overcoming attractions between them.
Questions could include:
- What does the string represent? Fractions of oil or hydrocarbon molecules.
- Why are the strings different lengths? Because they contain different numbers of carbon atoms.
- Why does the longer one take more effort/energy to remove? It has more points of contact with other molecules, so more energy is needed to overcome the attractions between the particles (that’s why it needs a higher temperature to vaporise).
I use another model to demonstrate fractional distillation with different lengths of strings, by cutting about 20 pieces to a variety of lengths. Place the strings in an opaque plastic beaker with the free ends hanging over its edge. Push the other ends into the beaker so the ends tangle together. Ask some students to each loosely take hold of a string end and then try to remove it. The longer the string, the more tangled it will likely be, and harder to pull out.
Explain that the different lengths of strings represent different fractions of oil with varying chain lengths. The longer chain molecules have more points of contact and so require more energy to separate them from the mixture, so the temperature will be higher. You can tie knots in string pieces to represent carbon atoms. The shortest string would have one knot and the longest string the most knots (evenly spaced, ideally). This helps the strings tangle together, becoming more difficult to separate.
Ask students to repeat the exercise and to explain what’s happening. You could give some key vocabulary or ask scaffolding questions. You can also use Molymods to build models of alkanes with different lengths, muddling them together in a Gratnells tray. Be clear that we are not breaking bonds within the molecules but rather overcoming attractions between them.
You could ask students what the string represents (fractions of oil or hydrocarbon molecules), or why the strings vary in length (because of the different numbers of carbon atoms). You could also ask why the longer one is harder to remove (due to more points of contact with other molecules, so more energy is needed to overcome the attractions between the particles).
By verbalising the process, students can practise their answers before committing them to paper in response to prompts like: explain why longer chain hydrocarbons come off the fractionating column at the bottom of the column.
These models are relatively simple, and are easy to prepare and use. They’re effective because students gain understanding of what each part of the model represents and they can use that knowledge to describe the concept, or process represented, for themselves.
Sarah Longshaw












No comments yet