Try these teacher-tested tips and ideas to engage learners when teaching atomic structure and radioactive decay
No one has ever seen inside an atom, so to understand atoms in the classroom, students build models and draw diagrams. Using playdough to build atomic structures is cheap, colourful and tactile, which makes it fun and engaging for learners.
Playdough recipe
You need:
- one cup of flour
- two teaspoons of cream of tartar
- half a cup of salt
- one tablespoon of vegetable oil
- one cup of water
- food colouring – gel colours work best
Put everything into a microwaveable bowl and mix well. Microwave on high for 30 seconds, then mix again. Keep repeating this step. Be patient, because after the first couple of minutes you get a lumpy mess. Keep going and eventually it will come together. One last 30 seconds will ensure it is firm and not sticky. Leave the playdough to cool before storing in an airtight tub.
While playdough is generally safe, consider if any students have gluten allergies and provide an alternative modelling material. Two or three quantities of the above mixture are enough for a class of 30 to build model atoms in small groups.
I first used playdough to get pairs of 12 year-olds creating models of atoms. I gave them a small amount of playdough in two different colours and a slip of paper with the details of an element from the periodic table – the first three periods work well as anything bigger than argon gets unwieldy. I wanted to see if the students understood the difference between atomic number and mass number and if they could work out the correct number of neutrons and electrons. I told the pairs to use the same colour for protons and electrons (charged particles) so it was clear which were the neutrons.
Using this approach, you can see what students are thinking as they work and identify any misconceptions or confusion. You can then provide feedback on the spot or identify class-wide ideas you need to revisit. For example, after I had a quick discussion with one group about why electrons aren’t included in the mass number, they changed their model’s electron size.
Building understanding
You can develop this activity by asking learners to guess which element other groups have built by looking at their atomic model. Can they find the correct atom in the periodic table by counting the numbers of subatomic particles and identifying the atomic (not mass) number?

Dig out the playdough when revisiting atomic structure with your 14–16 learners. Get them to draw electron shells with whiteboard pens and populate them with electrons. Can they build ions and identify each other’s models? Can they build isotopes – what changes and what doesn’t?
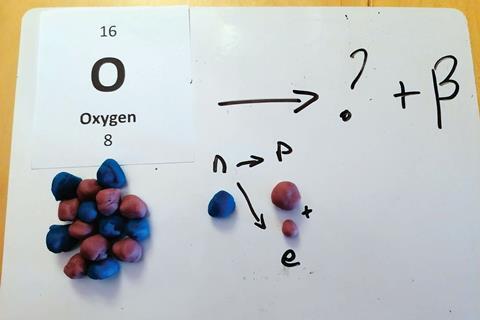
Playdough modelling lends itself well to demonstrating processes that change. I have used it to model alpha and beta nuclear decay, as learners often find it confusing to work out where the radioactivity is coming from. For example, students used playdough models to move nucleons from one side of a nuclear equation to the other to work out the unknown product of alpha decay.
Students also swapped a neutron from an oxygen atom for a proton and an electron to show that the electron (beta particle) is emitted from the nucleus in beta decay and not the outer shell.
Tips
There are drawbacks to using playdough. Students will be tempted to play with it and it will get stuck in crevices, trodden into the floor, made into other models or just disappear. You can mitigate these potential issues by giving clear instructions and expectations. You will know how best to manage your own practical classes.
Get learners to build on mini whiteboards as this will help to limit how big they build the atoms. You can also move the models to a visualiser and easily wipe down whiteboards if the dough gets sticky.
Having something to play with engages less confident learners and allows them to access the activity until you can work with them one to one. Enjoy modelling!
More modelling ideas
Explore these additional teaching ideas that use readily-available resources to model chemical concepts.
- Use plasticine to model ionic bonds in your classroom.
- Teach students to model periodicity with materials they have in their homes.
- Get students out of their seats and working with their hands to teach polymerisation and fractional distillation.
- Build understanding of atomic structure at 11–14 to help learners focus on higher-order thinking skills and achieve later on in school.
Eleanor Clapp
Find more modelling activities online: rsc.li/4chedkT











No comments yet