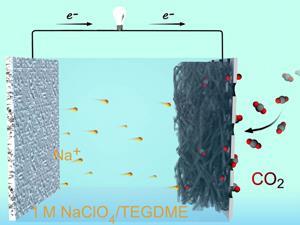
New cathode material makes for energetic, rechargeable sodium–carbon dioxide batteries that could power Mars vehicles
Rechargeable first for promising battery tech was first published by Chemistry World.
New cathode material makes for energetic, rechargeable sodium–carbon dioxide batteries that could power Mars vehicles

Rechargeable first for promising battery tech was first published by Chemistry World.
No comments yet