Prevent misconceptions and tackle understanding gaps at 14–16 with these essential teaching strategies

To properly understand the sustainable manufacture of many products in industry, students need to comprehend the concepts associated with chemical equilibrium. When reactions are reversible, for example, it’s important for manufacturers to optimise reaction conditions to produce as much of the desired product as possible. They must also minimise the quantity of starting materials, identify reactions with high atom economy and limit the amount of waste material. Manufacturers also need to consider the energy demands of high temperature and pressure reactions.
A well-known and commonly studied industrial reaction is the production of ammonia from hydrogen and nitrogen, the Haber–Bosch process. Industrial production of ammonia allows the mass manufacture of chemical fertilisers, which in turn increases crop yields and helps to meet the needs of a growing population. The process also provides a potential ethical dilemma because ammonia is a precursor for the synthesis of numerous explosives.
Resources for your classroom
- Support higher-attaining students with the practical work and consolidation tasks from this resource pack, looking at the impact of changing conditions on equilibriums.
- Give post-16 learners these questions to challenge prior conceptions of equilibriums and Le Chatelier’s principle.
- When discussing industry context with your students, point them to a less energy-intensive process than the Haber–Bosch which could slash carbon dioxide emissions from fertiliser production.
What students need to know
We can use Le Chatelier’s principle to make simple qualitative predictions about how equilibrium reactions will respond to changes in conditions. The principle is that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change and re-establish an equilibrium. To put this more simply, when changes are imposed on a system, the position of equilibrium alters to oppose this change. This principle allows us to predict the effects of changes in temperature, pressure and concentration on the yield of a reversible reaction.
At 14–16, students learn the qualitative interpretation of Le Chatelier’s principle, where they can easily develop misconceptions. For example, learners can wrongly think an exothermic reaction would be favourable after an increase in temperature because releasing energy would minimise the system change.
Use reversible chemical reactions with visible changes to show students how the yield is affected
Also, students are often taught rates of reaction directly before equilibrium. When describing situations, they confuse the rules for rate with equilibrium, causing further misunderstanding. You can separate these topics to minimise this problem.
Bridging the gap to post-16 study
Post-16 courses recap the principles studied at 14–16. Students then move on to a more quantitative understanding of chemical equilibrium. Central to this is the definition and calculation of equilibrium constants, and how these constants help describe positions of equilibrium.
A key point about the equilibrium constant is that it is constant at all concentrations and pressures, and only temperature affects this value. The equilibrium constant, in terms of concentration, for the reaction aA(g) + bB(g) ⇌ cC(g) + dD(g) is defined by the law of chemical equilibrium as:
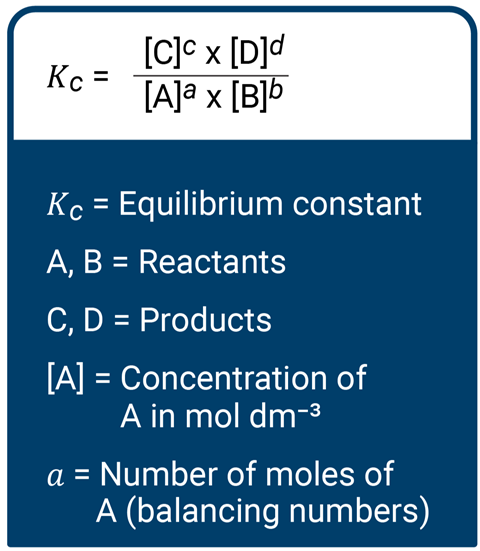
Developing the equation
In order to use this equation, the reaction must be homogeneous (ie all substances must be in the same phase), there must be dynamic equilibrium, and the concentrations of each substance at equilibrium must be clear. The value of Kc determines the favoured side of the equation, and therefore the relative proportion of species in the mixture. So, if Kc is much greater than 1, the mixture contains mainly products; if Kc equals 1, the equilibrium will lie midway between reactants and products; and if Kc is much less than 1, the mixture contains mainly reactants.
We can use the Haber–Bosch process in a worked example to show this. The task could be to determine the equilibrium constant for a mixture of 0.030 mol dm-3 N2, 0.037 mol dm-3 H2 and 0.016 mol dm-3 NH3 at a constant temperature, given the equilibrium N2(g) + 3H2(g) ⇌ 2NH3(g).
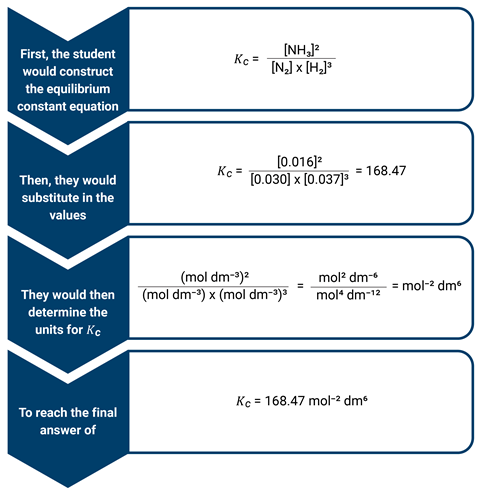
When conditions change in the reaction, students can then use these mathematical expressions to explain how the change in position of equilibrium occurs. For example, if ammonia was removed from the equilibrium, then the value of the numerator of the Kc expression decreases. This causes the value of the Kc to decrease. To restore Kc to its original constant value, the numerator must increase, and the denominator decrease. In other words, the concentrations of N2 and H2 must decrease (be used up) and increase for NH3 (be produced). So, the forward reaction is favoured, the position of equilibrium shifts right and the dynamic equilibrium is re-established.
This change can also be described in terms of rates of reaction. When a closed system is in dynamic equilibrium, the rates of the forward and backward reaction are equal. However, when the concentration of NH3 is decreased, the rate of the reverse reaction decreases. In that instant, however, the rate of the forward reaction remains the same. The forward reaction is then higher than the reverse, which increases the concentration of NH3 and re-establishes the equilibrium.
Suggestions for teaching
- Explain simple changes in conditions of dynamic equilibriums using Le Chatelier’s principle as a useful qualitative model.
- Use chemical reactions with visible differences in changing conditions to show students how the yield is affected
- Students need to practise applying Le Chatelier’s principle to numerous chemical reactions. Slowly increase the difficulty of the equation or scenario; for example, start with chemicals labelled as letters rather than their formulas.
- Use equilibrium constants as a quantitative method to describe reactions at dynamic equilibrium.
Emma Owens is an assistant subject leader of science at Horizon Community College














No comments yet