Use these ideas and resources to help students understand conjugated pi systems
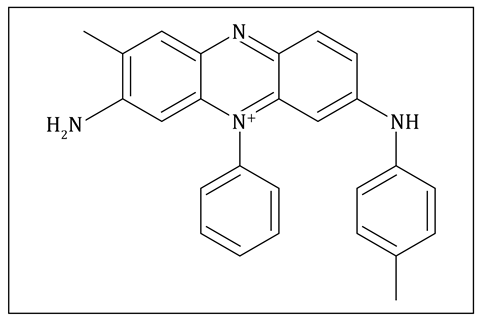
We live in a colour-filled world. A chromophore is a molecule which absorbs light of a particular wavelength and reflects colour as a result. We commonly refer to chromophores as coloured molecules. These molecules often have large sections of alternating single and double bonds. Where adjacent pi bonds are parallel to one another, they can merge or link together to form extended molecular orbitals that we call conjugated pi systems. The conjugated pi systems can interact with longer wavelengths of light, which is how some of them produce colour. Mauveine, also known as Perkin’s mauve, is a chromophore and was one of the first synthetic dyes. It has conjugated pi systems that interact with longer wavelengths of light and produce a mauve colour.
Download this
Lesson plan for age range 16–18
Explore the structure of benzene using this lesson plan, including student worksheet, slides and information cards.
Download the resource from the Education in Chemistry website:
What students need to know
Aromatic compounds are conjugated cycloalkenes that have enhanced stability because of the delocalisation of the electrons in the pi system. Benzene, C6H6, is the archetypal aromatic compound. It has a conjugated pi system with six electrons delocalised around the ring in a six-centre pi orbital.
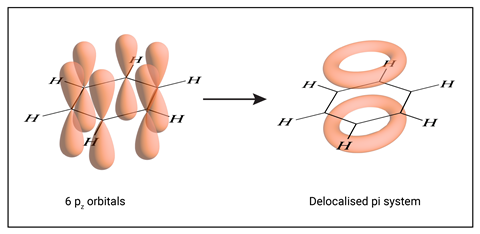
The conjugated pi system extends around the whole ring rather than three isolated pi bonds in three sides of the hexagon. We often draw benzene with a circular ring to represent the pi system.
Both alkenes and aromatic compounds react with electrophiles which are attracted to the pi electron density. A conjugated pi system has greater thermodynamic stability than isolated pi bonds. This affects the reactions of aromatic compounds which undergo electrophilic substitution rather than the electrophilic addition reactions of alkenes. Stronger electrophiles are needed to react with benzene than with alkenes.
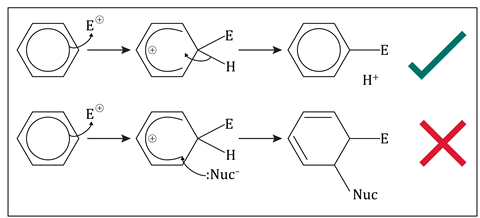
The mechanism of electrophilic substitution at a benzene ring involves the formation of a carbocation intermediate with the positive charge spread around a five-carbon delocalised pi system with four electrons left in it. We get this electrophilic substitution as the delocalised pi ring is restored.
We do not get electrophilic addition as this would break up the pi ring and lose the extra stability it confers.
In these mechanisms E+ represents an electrophile, :Nuc- represents a nucleophile.
Common misconceptions
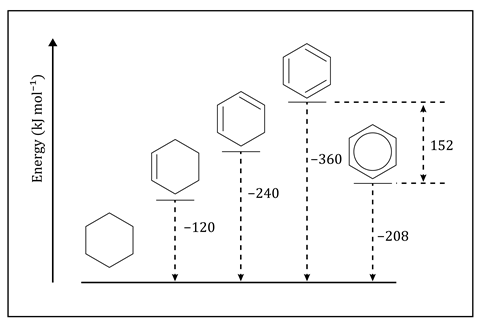
Most teachers start this topic with a comparison of the structure that Kekulé proposed for benzene – cyclohexa-1,3,5-triene – and a structure with a delocalised pi ring. Ensure that students understand the extra thermodynamic stability that benzene, with its delocalised pi ring, has compared to the theoretical cyclohexa-1,3,5-triene. Enthalpy diagrams, such as the one here which shows predicted and real hydrogenation enthalpies, help but can contribute to the misconception that when we draw Kekulé’s structure we mean the theoretical cyclohexa-1,3,5-triene rather than benzene. Students can think that the resonance structures represent distinct entities rather than a single concept. Remind learners these structures are representations, not reality.
Another common misconception about the mechanism of electrophilic substitution is that the carbocation reactive intermediate is electron deficient at the carbon which has formed a new bond to the electrophile.
Ideas for your classroom
Before you show your students the Kekulé structure for benzene you have an ideal opportunity to introduce the concept of double bond equivalents, DBE, to help with structure determination. Both double bonds and rings reduce the number of hydrogens in a molecular formula by two. Hexane C6H14 has no double bonds. Cyclohexane and hexene both have the formula C6H12, each has one DBE. Benzene C6H6, has four DBE. Download the accompanying resource for an entertaining question on DBE.
At the start of this topic, (re)teach how pi bonds are made from the overlap of parallel p orbitals. Point out to students that the resulting pi orbitals have electron density above and below the sigma bond between the two atoms.
Evidence for delocalised structure of benzene
Because of the hazards associated with benzene students will not be handling it, so introduce context. Tell the story of Kekulé proposing his structure for benzene after dreaming one night of a snake swallowing its own tail.
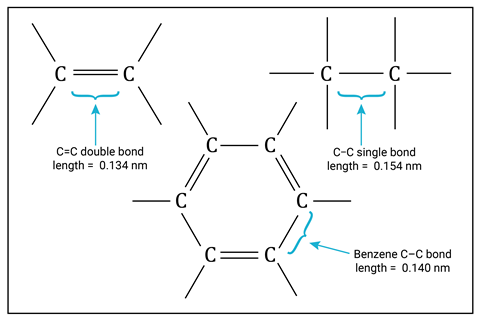
Point out that the bond lengths in benzene are all the same and intermediate between the longer C–C single bond and the shorter C=C double bond.
Electron density maps provide evidence of this bond length by enabling us to measure the distance between nuclei.
Physical models or good diagrams can help students understand that the pi molecular orbital in benzene goes around the whole ring.
Explain that with this six-electron six-centre pi system, students are moving beyond covalent bonds that are simply two shared electrons.
Use the thermodynamic evidence against cyclohexa-1,3,5-triene to explore students’ grasp of thermodynamic stability. The electrons could occupy the space of three separate pi bonds but the transformation into a delocalised pi ring is exothermic so that’s what the electrons do. Like a ball rolling down to the bottom of a hill. Link this to the relative stability of the carboxylate ions compared to the alkoxide ions, if you have covered this topic.
More ideas
When teaching about nitration, explain the outline method of producing TNT (trinitrotoluene) and how each successive nitration requires a higher temperature as each NO2 group deactivates the ring.
Revisit the structure of graphene – a single layer from graphite. Show learners how they can now explain the delocalised electrons between layers they met earlier as electrons in a conjugated pi system that extends across the whole layer.
When teaching acylation or alkylation, emphasise the importance of these reactions as a means to change the carbon skeleton in synthetic routes.
More resources
- Use a quick practical to reinforce the link between aromatics and chromophores with the microscale synthesis of azo dyes. In this experiment, students prepare an azo dye and use it to dye a piece of cotton.
- Find more questions on aromatic chemistry in this Starter for 10 activity.
- Check and revise your learners’ understanding of organic chemistry with this multiple choice Organic synthesis quiz.
- For more context, this science research news story investigates benzene reactions in fizzy drinks.
More resources
- Use a quick practical to reinforce the link between aromatics and chromophores with the microscale synthesis of azo dyes: rsc.li/43buWo0
- Find more question on aromatic chemistry in this Starter for 10 activity: rsc.li/4kczH6Q
- Check and revise understanding of organic chemistry with this multiple choice Organic synthesis quiz: rsc.li/3YX5Nea
- For more context, this science research news story explores benzene reactions in fizzy drinks: rsc.li/4mrKDyQ
Checking for understanding
Discuss the number of NMR signals in substituted benzene compounds and how activating and deactivating groups make the benzene ring more or less reactive with your class. Ask students to explain why we get substitution rather than addition at a benzene ring, why the delocalised pi ring is more stable than three pi bonds and why we need Friedel–Crafts catalysts to get benzene to react with halogens. Finally check learners understand how Friedel–Crafts catalysts work in the alkylation and acylation reactions of benzene and get students to put the curly arrows into words for mechanisms.
Discuss the number of NMR signals in substituted benzene compounds and how activating and deactivating groups make the benzene ring more or less reactive with your class. Ask students to explain why we get substitution rather than addition at a benzene ring, why the delocalised pi ring is more stable than three pi bonds and why we need Friedel–Crafts catalysts to get benzene to react with halogens. Finally check learners understand how Friedel–Crafts catalysts work in the alkylation and acylation reactions of benzene and get students to put the curly arrows into words for mechanisms with this previous post-16 CPD article (rsc.li/439ED4U).
Take-home points
- The extended pi bond around a benzene ring moves students’ understanding forward from two-centre two-electron bonds to encompass conjugated pi systems.
- These systems show greater stability than they otherwise would because the electrons are able to spread out further from each other.
- This understanding links to many important topics including the relationship between reactivity and thermodynamic stability.
Article and resource written by Tim Jolliff, a science education consultant and former 11–18 chemistry teacher















2 readers' comments