Everything you need to help your 14–16 learners with this tricky topic
Equilibrium is an important process in industry, eg for making ammonia using the Haber process which is a reversible reaction. Manufacturers want to make as much product as possible, minimising the quantity of starting materials left over. They also want to minimise energy use when conducting reactions at high temperatures and pressures.
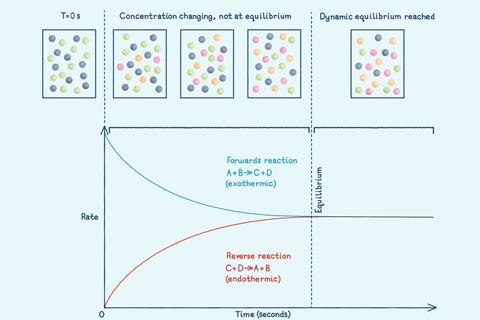
To make reversible chemical reactions as efficient and sustainable as possible, manufacturers need to understand equilibrium. Because the equilibrium position – the concentrations of substances present at equilibrium – affects the yield of the product.
Reversible reactions
Most chemical reactions are irreversible, eg combustion, where the reaction only takes place in one direction.
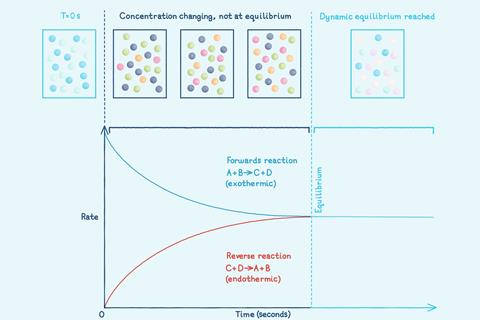
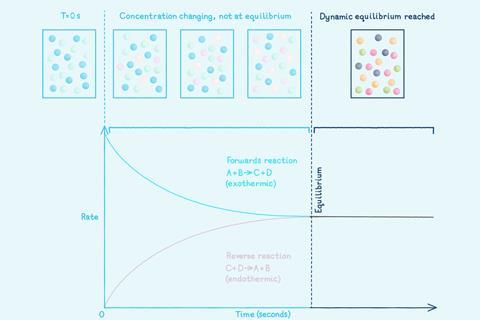
However, many chemical reactions are reversible: the products can react together to reform the original reactants. A reversible reaction is represented by a double arrow symbol: ⇌.
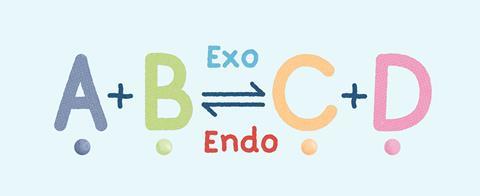
Download this
Infographic poster, fact sheet and student worksheet. Display the poster in your classroom or on a projector. Alternatively, print it and use as a handout.
The accompanying storyboard activity asks learners to describe and sequence a dynamic equilibrium reaction using words and images.
- Poster as pdf
- Fact sheet as MS Word or pdf
- Fully scaffolded student worksheet as MS Word or pdf
- Partially scaffolded student worksheet as MS Word or pdf
- Unscaffolded student worksheet as MS Word or pdf
- Presentation slides including hints and answers as MS Powerpoint or pdf.
- Teacher guidance and answers as MS Word or pdf
More resources
- Use these tips, strategies and teaching ideas when covering these reversible reactions and equilibrium at 14–16
- Demonstrate equilibrium reactions with these class practicals and teacher demonstrations: the reversible reaction of hydrated copper(II) sulfate, as given as an example in this article; demonstrate the equilibrium between two different coloured cobalt species or use this copper-based, colour-changing demonstration to introduce your 14–16 learners to reversible reactions.
- Tie sustainable industry into your chemistry topics and complement your teaching of reaction kinetics, catalysts and equilibrium with UN sustainable development goal 9.
Did you know …?
In 2020, the Haber process – a reversible reaction – produced over 176 million tonnes of ammonia for fertiliser and other uses.
Get more posters for 14–16 year-old learners
Download these posters and the associated classroom activities to teach these topics in your classroom:
Downloads
Equilibrium poster
PDF, Size 5.05 mbEquilibrium and reversible reactions fact sheet
Handout | PDF, Size 0.17 mbEquilibrium fully scaffolded student worksheet
Handout | PDF, Size 0.54 mbEquilibrium partially scaffolded student worksheet
Handout | PDF, Size 0.43 mbEquilibrium unscaffolded student worksheet
Handout | PDF, Size 0.54 mbEquilibrium presentation with answers
Presentation | PDF, Size 1 mbEquilibrium teacher guidance and answers
Handout | PDF, Size 0.43 mbEquilibrium and reversible reactions fact sheet
Editable handout | Word, Size 0.44 mbEquilibrium fully scaffolded student worksheet
Editable handout | Word, Size 0.71 mbEquilibrium partially scaffolded student worksheet
Editable handout | Word, Size 0.66 mbEquilibrium unscaffolded student worksheet
Editable handout | Word, Size 0.71 mbEquilibrium presentation slides with answers
Editable handout | PowerPoint, Size 2.56 mbEquilibrium teacher guidance and answers
Editable handout | Word, Size 0.62 mb














2 readers' comments