Adam Boxer believes we should rethink how we order the teaching of atomic structure, bonding and groups 1, 7 and 0
The start of 14–16 chemistry is a beautiful thing. Having conceived of atoms as billiard balls for the first few years of secondary school, we are finally free to start teaching students about the power of the electron shell model of the atom. A simplification, to be sure, but a simplification which explains the elegance of the modern periodic table, Mendeleev’s foresight, how atoms interact and bond with each other, how simple and giant structures come to be, the trends in reactivity in groups 1 and 7 and the nobility of group 0.
The power of the process
When the substance of our lessons is so deeply interrelated, it’s crucial we do it in the right order, so sequencing is everything. Get it wrong, and students’ knowledge becomes fragmented and disjointed, untethered from a unifying narrative. Get it right, and students will appreciate the interconnectedness of chemical concepts and how mastery of a few basic principles can unlock a vast range of applications.
The key stage 4 national curriculum begins with embedding knowledge of atomic structure and the periodic table (including trends in groups), before moving on to knowledge of structure and bonding. Several exam specifications do the same, discussing groups 1, 7 and 0, before doing structure and bonding. Popular textbooks often follow suit, resulting in this order:

atomic structure > periodic table > groups 1, 7 and 0 > structure and bonding
I find this sequence problematic, due to the aforementioned risks of knowledge fragmentation. The problem arises from the trends in reactivity in groups 1 and 7 and the inert nature of group 0. When we teach reactivity at 14–16, we say that group 1 elements become more reactive down the group due to the ease of losing the outer shell electron, and group 7 less reactive down the group due to the difficulty of gaining an outer shell electron.
These explanations only make sense to students once they have studied the formation of ions and the behaviour of outer shell electrons. Similarly, for group 0, explaining that the elements need to neither gain nor lose electrons is far easier once students have fully internalised outer shell behaviour.
Mastery of a few basic principles can unlock a vast range of applications
To mitigate this problem, some textbooks go so far as to add the formation of ions into the sequence earlier on:

atomic structure > periodic table > atoms into ions > groups 1, 7 and 0 > structure and bonding
This seems to put the cart before the horse. The decision has been made to do groups before structure and bonding, and therefore we shoehorn other topics in to make it work. Far better to abstract ourselves from the need to follow a specification. In this case, I therefore advocate:
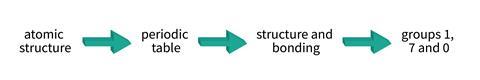
atomic structure > periodic table > structure and bonding > groups 1, 7 and 0
Think ahead
When it comes to the final section, students will need a strong understanding of outer shell completion, and fluency in the charges of subatomic particles and their electrostatic behaviour. As such, you will want to make sure you ask questions earlier on in the sequence, like:
- What is the charge on a nucleus?
- What is the total charge on the nucleus of Na/P/Br …?
- Explain why nucleuses and electrons are attracted to each other.
You can also foreshadow later topics by asking questions such as:
- Draw an atom of neon. Explain why it does not need to gain or lose electrons.
- Draw an atom of helium. Explain why it does not need to form ions.
When it comes to the final section, students will need a strong understanding of outer shell completion, and fluency in the charges of subatomic particles and their electrostatic behaviour. As such, you will want to make sure you ask questions earlier on in the sequence. For example, ask students what the charge on a nucleus is, what the total charge on the nucleus of Na/P/Br … is and to explain why nucleuses and electrons are attracted to each other.
You can also foreshadow later topics by asking students to draw an atom of neon and explain why it does not need to gain or lose electrons, for example. Or get them to draw an atom of helium and explain why it does not need to form ions.
Despite not having studied group 0, once students have studied outer shells and ions, they should be able to answer questions like this – albeit not to an exam-ready standard. You can then return to them and refine their answers at a later stage.
This is just one example of where departing from a traditional or specification-determined order can mean that students attain a more sophisticated and holistic comprehension of fundamental chemistry than otherwise.
More like this
Here’s how to sequence your teaching of atomic structure and periodicity for post-16 students.
Adam Boxer









3 readers' comments