Use these teaching ideas to help students master these key chemistry concepts and overcome misconceptions
Getting to grips with atomic structure helps students understand how and why elements interact with others (or not) and their specific locations in the periodic table.
The periodic table allows chemists to successfully predict the properties of previously undiscovered elements, and go on to manufacture them, an important concept for a sustainable world that relies on SMART materials and technology.
Most students – and some teachers – underestimate the wealth of knowledge within the periodic table. One of my best teaching tools was a full-scale periodic table on a classroom wall with hand-painted chemical symbols, masses and atomic numbers on A4 pages. It was perfect for teaching molecular mass, moles, electronic arrangement, valency and periodic trends.
Atomic structure and periodicity are the two topics that provide the foundation to any level 3 chemistry specification, as reflected in the allocation of 28% of the marks in a 2022 AS-level chemistry paper.
What students need to know
Students should be able to determine the number of protons, electrons and neutrons for any given atom, isotope and ion of any element in the periodic table. Introduce nuclear attraction, atomic and ionic radius and the trends for reactivity and radiuses here. Emphasise the role of electrostatic attractions within the atom in keeping the subatomic particles together.
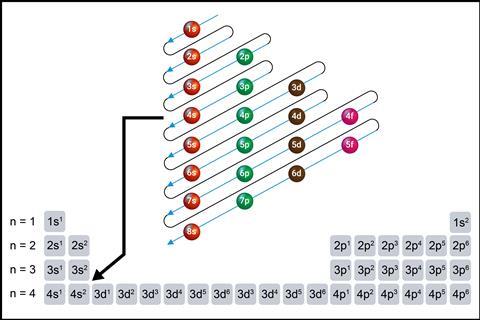
It’s essential for students to understand how electrons are arranged in shells, subshells and orbitals, and the order orbitals are filled. The effects of electron spin and determining electronic arrangements for the transition metals and the arrangements considered as more stable are key ideas. This then leads logically into trends in ionisation energies, electron affinities, melting and boiling points.
Common misconceptions
- Atoms need to gain full outer shells of electrons to be stable.
- Electrons are stationary.
- Nuclear attraction is one way (from the nucleus to the electrons).
- The number of electrons that an atom loses or gains determines the number of ionic bonds that it can form. For example, sodium can form one ionic bond with chlorine.
- The atomic nucleus is the control centre for the atom.
- The protons in the nucleus attract one electron each.
- The electrons repel the nucleus.
- Periodic trends definitions and trends across periods.
Ideas for your classroom
Before introducing the topic of atomic structure, consider working backwards. Measure 64 g of copper filings into a beaker and ask:
- Why did I choose 64 g?
- How many atoms are there in the beaker (introduce Avogadro’s constant)?
- Can you now calculate how many protons, neutrons and electrons are in the beaker?
Get the class to draw one of the copper atoms to uncover any misconceptions and eliminate them using atomic models and the solar system analogy.
Before introducing subshells and orbitals, assess prior knowledge with diagnostic testing to determine your starting point. Subshells, atomic orbitals and energy levels can be a huge step up from level 2 for some students. Emphasise that electrons are constantly in motion, with various degrees of energy and that an orbital is simply a region that represents the position the electron occupies most when in motion. This video of Gryzinski’s free-fall atomic models for chemical elements will help learners visualise this motion.
Before introducing subshells and orbitals, assess prior knowledge with diagnostic testing to determine your starting point. Subshells, atomic orbitals and energy levels can be a huge step up from level 2 for some students. Emphasise that electrons are constantly in motion, with various degrees of energy and that an orbital is simply a region that represents the position the electron occupies most when in motion. This video of Gryzinski’s free-fall atomic models for chemical elements (bit.ly/40pDHYk) will help learners visualise this motion.
Download this
Electron configurations infographic and resources, for age range 16–18
Help your post-16 students understand orbitals and shells by asking them to correct and improve incorrect electron configurations.
Download the poster, fact sheet and peer-assessment activity from the Education in Chemistry website: rsc.li/46SyURH
To write electron configurations correctly, students must be aware of the various energy levels of the shells surrounding the nucleus, the number of electrons in each subshell and orbital, and the order they are filled.
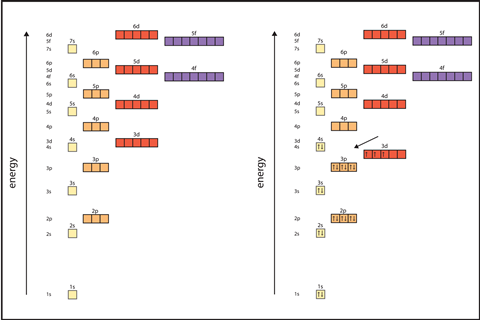
When you need to transition into the overlapping of the 4s and 3d orbitals, use the periodic table to show students how the 4s electrons are filled before an empty 3d. Remind them that 3d orbitals with electrons in them are lower in energy than the 4s orbitals and come before the 4s orbital in configurations.
When teaching the periodic trend in ionisation energy, I like to hand out a blank periodic table and ask the class to write the electronic configuration of the outermost shell for the elements in period 3. Once complete, I provide the values for the period 3 elements and ask students to compare the graph with the electron arrangement, suggesting reasons why the energies vary.
You could carry out a similar activity for melting and boiling points – ask students to label the blank table’s elements as metals or non-metals, and as solids, liquids or gases. I also recommend these modelling ideas.
You could carry out a similar activity for melting and boiling points – ask students to label the blank table’s elements as metals or non-metals, and as solids, liquids or gases. I also recommend these modelling ideas: rsc.li/3Qz5AJc.
More resources
- Help post-16 students consolidate their ideas about atoms and atomic structure by working in pairs to identify key concepts.
- Quiz your students on periodic table trends, such as melting points, ionisation energies and atomic radiuses.
- Assess students understanding of prior learning by revisiting the topic of atomic structure.
- Display this poster in your classroom before tasking 14–16 year-old learners to draw electron configuration diagrams using the resource’s easy-to-follow steps.
- Do some follow-up some work with your students on the reactivity trends in groups 1 and 7.
Checking for understanding
Assessment for learning can be most effective when students ‘create a change in their long-term memory’. So, assessment activities should help students access prior knowledge, motivate them to study, help identify gaps in knowledge, and reveal and challenge misconceptions. Spaced learning has also been shown to be effective so assess knowledge periodically to improve students’ long-term memories. The atomic structure starters worksheet and chemical misconceptions resources have been designed to do so, and this revision map chapter from the misconceptions collection is perfect for assessing knowledge of the periodic table.
Assessment for learning can be most effective when students ‘create a change in their long-term memory’ (bit.ly/3smInSg). So, assessment activities should help students access prior knowledge, motivate them to study, help identify gaps in knowledge, and reveal and challenge misconceptions. Spaced learning has also been shown to be effective and so assess knowledge periodically to improve students’ long-term memories. Use the atomic structure starters worksheet (rsc.li/4730Sue) and chemical misconceptions resources (rsc.li/472tl3a) to effectively assess knowledge.
Take-home points
- Atomic structure and periodicity provide a solid foundation to any level 3 chemistry class.
- Scaffold learning, embed comparative and half-completed models, and use strategic assessment activities to build long-term memory.
- Make use of free high-quality resources to eliminate misconceptions, practise identifying subatomic particles, writing electronic configurations and understanding periodic trends.
Johanne Brolly is an ex-teacher and education coordinator in Ireland for the RSC












No comments yet