Demonstrations designed to capture the student's imagination
Demonstrations designed to capture the student's imagination, by Adrian Guy of Blundell's School. In this issue: explosive nitrated carbon compounds
Guncotton is trinitro-cellulose, or cellulose nitrate. The material is used as a propellant and as a blasting explosive. Guncotton burns at a lower temperature than gunpowder - a mixture of sulfur, charcoal and potassium nitrate - but produces several times the quantity of gas (comprising CO, CO2, H2O, N2), leaving almost no residue and little smoke.
Nitrated carbon compounds
A partially nitrated version of guncotton (pyroxylin) can be made easily and relatively safely in the laboratory by adding a nitrating mixture of sulfuric and nitric acids to naturally occurring cellulose - cotton wool. The resulting material suitably demonstrates the explosive nature of nitrated carbon compounds.
Kit
- 250 cm3 Beaker, ice bath;
- 20 cm3 Conc. sulfuric acid, H2SO4;
- 10 cm3 Conc. nitric acid, HNO3;
- Universal indicator; cotton wool;
- Sodium carbonate powder;
- Stirring rod, thermometer, filter paper, tongs, chemical-resistant gloves, protective face mask.
Procedure
This procedure must be done in a fume cupboard. To protect against chemical splash or explosions a protective face shield and chemical-resistant gloves must be worn.
To make the nitrating mixture first cool 20 cm3 concentrated sulfuric acid to 5°C in a 250 cm3 beaker in an ice bath. Then, slowly, with stirring, add 10 cm3 concentrated nitric acid, ensuring the temperature remains below 10ºC. Leaving the nitrating acids in the ice bath, slowly, and with care, add ca 0.5g of clean, dry cotton wool, ensuring the mixture doesn't rise above 20ºC. Do not allow the cotton wool to remain in the mixture for more than an one hour at <5 ºC as there is an increased risk of slow formation of more nitrated product which could be illegal under the Explosives Regulations 2014. As the material is nitrated it takes on a more slimy texture and a light brown colour. The resulting nitrocellulose must be neutralised and washed.
Using tongs, remove the nitrated cotton from the acid mixture, squeeze out as much excess acid as possible and carefully place the material in a large ice-water bath. I use a water trough. To this add 2 cm3 of universal indicator solution, which turns the bath a bright red colour because of the acidity.
Sprinkle sodium carbonate powder into the bath and stir until the solution turns green, and is now neutral and safe to handle. Stir well to expose all the nitrated cotton to the sodium carbonate solution. The material will also turn green/orange when neutral. (Note: large quantities of sodium carbonate are required.)
Finally, remove the nitrated cotton from the solution and wash in cold, clean water, removing any solid sodium carbonate. 'Fluff up' the material so it resembles cotton wool and place on a piece of filter paper in a warm, dry place for 24 hours or until dry.
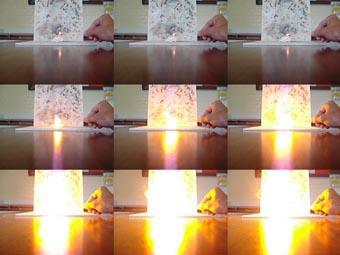
For the demonstration wear safety goggles and tie back long hair. First ignite a piece of untreated cotton wool on a heat-resistant mat. The cotton wool burns slowly, producing smoke and charred remains. Then take a small piece of your guncotton, place it on a heat-resistant mat and ignite with a lit splint. The material should burn rapidly with a bright orange flame. If your guncotton is suitably nitrated and, more importantly, dry there should be no residue left on the mat and the reaction should be over in a fraction of a second.
Safety
This demonstration should only be done if you are familiar with the nitration of organic compounds since there are considerable risks associated with all stages. Concentrated nitric and sulfuric acids are corrosive and contact with the skin or eyes can cause serious damage. Concentrated sulfuric acid is a strong dehydrating agent and generates heat when diluted with water. If spilt on skin or bench, mop up excess acid with a dry cloth/tissue then wash with lots of water and treat with sodium hydrogen carbonate solution. Pour excess nitrating mixture into a large excess of cold water. The guncotton is explosive when dry and there is an extreme risk of explosion by shock, friction, fire or other sources of ignition. The material must not be confined nor must it be stored. Destroy any left after the demonstration by burning safely in small quantities. CLEAPSS have an updated version of this demonstration which differs slightly from this version.
Teaching goals
This demonstration reinforces the nitrating procedure studied at A-level. The nitronium ion (NO2+) generated in the acid mixture nitrates the alcohol groups on the cellulose to form the ester group -CONO2.
The nitronium ion is formed by the action of sulfuric acid on nitric acid. The nitric acid acts as a Lewis base and accepts a proton from the sulfuric acid, forming the intermediate H2NO3+, which decomposes to form the nitronium ion:
H2SO4 + HNO3 → HSO4- + H2NO3+
H2NO3+ → H2O + NO2+
The nitronium ion attacks one of the lone pairs of electrons on the oxygen of the OH group and the hydrogen is then removed by the HSO4- anion, leaving the nitro group attached and reforming the H2SO4 catalyst.









1 Reader's comment