With the development of sensitive analytical techniques, chemists can now search for unique trace markers in a person's breath to identify particular medical conditions. Breath analysis has the potential to become a non-invasive diagnostic tool in clinical practice.
-
There are ca 250 volatile organic compounds in a breath sample
-
Some compounds in people's breath have been linked to certain illnesses, eg diabetes and liver disease
Breath is a mixture of predominantly nitrogen, oxygen and carbon dioxide, plus some water vapour and inert gases, and trace amounts - parts per million (by volume) to parts per trillion - of volatile organic compounds (VOCs). Despite their low concentrations, these trace species reflect many processes occurring in the body. Endogenous (produced in the body) VOCs are the products of normal metabolism and of metabolic pathways that have altered owing to disease, and are transported via the bloodstream to the lungs where they are exhaled in breath.1 Analysis of breath could potentially provide a non-invasive means to assess a person's state of health.
Early clues
That breath odour is an indication of certain illnesses is not new. For many years we have known that the breath of diabetic patients, for example, has a characteristic fruity smell, owing to high levels of acetone (propanone) in the body. Insulin deficiency in diabetic patients means that their body glucose goes largely unused. As a consequence the body uses stored fat as an alternative source for energy, and acidic ketones build up. These ketones are detectable on the patient's breath.
The 'modern era' of breath analysis started around 40 years ago with studies notably by Linus Pauling and coworkers, which revealed the presence of 250 VOCs in an average breath sample.2 Another study by Michael Phillips and his group at the New York Medical College, US,3 found that breath composition varies widely from person to person, in terms of both the presence and abundance of VOCs. The researchers identified thousands of different VOCs in the breath of 50 healthy subjects, with only a small number of these compounds, ca 27, common to all subjects, probably indicative of the 'core' metabolic processes occurring in the body.
Breath sampling
One of the main advantages of breath analysis is that it can be used on people of all ages and conditions - from babies4 to patients on ventilators5 - and poses no risk to the patient. In theory, it should be possible to identify specific diseases, as well as monitor disease progression or response to treatment. However, breath presents an analytical challenge for chemists since it is transient, humid and, because the VOCs are present at trace levels, sensitive instrumentation is required. This, in turn, makes it difficult to reproduce and standardise any significant markers in breath.4

Moreover, breath is not a homogeneous sample. The first portion of an exhaled breath is the 'dead space' air which is located in the airways and does not participate in gas exchange. Following this is the 'alveolar' air from the deeper, gas-exchanging region of the lungs, which is in equilibrium with the blood. The alveolar air contains the highest concentration of blood-based VOCs, and is therefore the main target of most VOC analyses.
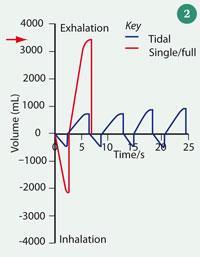
Superficially, sampling breath is both simple and quick, making it ideal for dealing with large numbers of patients. It can be collected and delivered directly into the analytical instrument in a single step or first collected and stored in a container (usually a polymer bag6 or canister7) for later analysis. The sampling 'apparatus' must be comfortable and safe for the patient, and easy to breath into. Disposable mouthpieces and bacterial filters are used to prevent contamination from one patient to the next. Fig 1 shows a breath sampling device, constructed of wide-bore components and including an in-line spirometer to measure breath volume. A spirometer trace is displayed in Fig 2. Plastic and rubber components that may themselves emit volatiles are avoided, and the body of the sampling devices and tubing are heated to prevent condensation of the breath on the internal walls, which is important since water-soluble VOCs could be lost by partitioning into the aqueous phase.
Measurement of VOCs
Much of the early work on breath analysis used off-line sampling and gas chromatography (GC)-based methods. GC provides good separation for the large number of VOCs and when coupled with mass spectrometry (MS), can be used to identify the VOCs based on their retention time and characteristic fragmentation patterns contained in the mass spectrum. The main disadvantage of GC or GC-MS is that pre-concentration is necessary and therefore data cannot be obtained in real-time. Pre-concentration is commonly achieved through sorbent traps, coated fibres (solid phase microextraction) or cryofocusing.8
More recently there has been a move to real-time sampling of breath, with a wide range of techniques both spectrometric and spectroscopic under development.9 Real-time sampling of breath affords several benefits, including the possibility of high-throughput screening, the ability to monitor patients in critical conditions, as well as reducing the number of sampling devices which are required for off-line sampling.
Two variants of direct MS - proton-transfer reaction mass spectrometry (PTR-MS)10 and selected ion flow tube mass spectrometry (SIFT-MS)11 - are currently being developed for real-time analysis of breath.
As with any sample being analysed by MS, the neutral compounds must first be ionised. In PTR-MS this is done by proton transfer of the gaseous sample inside a drift tube. The proton source is normally protonated water, H3 O+. Sample molecules and H3 O+ ions are introduced into the drift tube where proton transfer takes place for those VOCs with a greater proton affinity than water. Thus the major constituents of air and breath (nitrogen, oxygen and carbon dioxide) are not ionised, but many trace VOCs are readily protonated:
H3 O+ + VOC → VOCH+ + H2 O (1 )
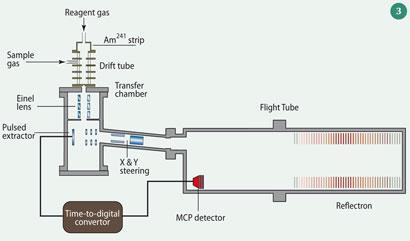
The fixed length of the drift (reaction) tube provides a fixed reaction time for the ions as they pass along the tube: the ion residence (and thus reaction) time can be measured or calculated. Under conditions where the proton donor (in this case H3 O+) concentration is largely unchanged by adding the sample, then measurement of the (proton donor)/(protonated acceptor) ion signal ratio allows the absolute concentration of the sample to be calculated. From the combination of reaction kinetics (or calibration) with MS, we can identify and measure individual organic gases on a relatively short timescale and with a sensitivity of ppt (by volume). A typical PTR-MS is shown in Fig 3.12 SIFT-MS is similar but pre-selects the reagent ion, and the reaction between proton donor and analyte occurs under low pressure field- free conditions.
Example measurements made with the PTR-MS instrument here at the University of Leicester are shown in Fig 4 and Fig 5. In Fig 4, the output from real-time analyses of a single breath are shown for the compounds acetone and acetonitrile (ethanenitrile). Acetone can be elevated by diabetes, while acetonitrile is associated with cigarette smoke inhalation. Acetonitrile from smoking is equilibrated among the bodily fluids (blood, total body water, and urine) and excreted via exhaled breath and urine.
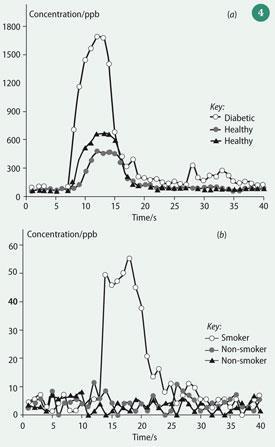
Fig 5 shows breath alcohol analysis using PTR-MS. Breath measurements were made both before and after the consumption of an alcoholic drink. This figure shows that there is an initial enhancement of ethanol as it is drunk ('mouth contamination') followed a secondary peak from the blood ethanol. The metabolic oxidation of the alcohol can then be followed in time through to acetaldehyde (ethanal) and acetic (ethanoic) acid.
Detecting disease
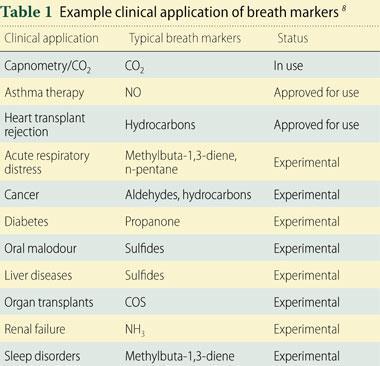
The fundamental aim of breath analysis is to identify VOC species and unambiguously attribute them to a specific disease. Some breath compounds have already been linked to particular medical conditions, though to date very few breath tests are in regular use in clinical practice. Table 1 lists some clinical applications of breath markers.8 The specificity of an individual marker for a disease state versus mapping multiple markers in terms of variance from the norm is an active area of research (see Box).13, 14
In contrast to measuring the 'naturally' occurring endogenous breath compounds we can also to detect a metabolite after administration of a drug or a test substrate. While the substrates vary depending on the nature of the investigation - eg gastrointestinal disorders, liver function, identification of Helicobacter pylori infection etc - the target metabolite is usually 13 CO2. For example, to identify the H. pylori infection, which is linked to the development of peptic ulcers, the patient takes a 13 C-urea solution. The H. pylori bacteria produce enzymes that metabolise urea to give carbon dioxide and ammonia. The measurement of increased levels of 13 CO2 in breath following the administration of 13 C-urea indicates the presence of infection.15 The advantage of this method is that is it analytically less challenging than looking for natural breath markers in the sense that you are looking for a specific and induced marker.
Future directions
Routine breath analysis is not yet part of the medical diagnostic armoury. We still need to be able to demonstrate that we can measure the trace VOCs with surety, sensitivity, simplicity and specificity in a clinical environment and we need more clinically sound evidence of the value of breath composition measurements as a screen for prognostic or diagnostic purposes.
There is no doubt that the technology will have to reduce in size and complexity to meet day to day clinical requirements.
In the future, breath analysis may well be deployed as part of a range of non-invasive diagnostic tests which give a holistic view of the patient's state.
Paul S. Monks is professor of atmospheric chemistry in the department of chemistry at the University of Leicester, Leicester LE1 7RH; Kerry Willis is a postgraduate student in the same department.
Breath analysis in organ transplantation
Diagnosis of acute rejection post lung transplant usually requires invasive biopsies, and patients have to be monitored for up to two years after the procedure. Researchers at the John Hopkins University School of Medicine in Baltimore, US, and at the City University of New York, US investigated the use of breath markers as a non-invasive alternative.14 The researchers screened post-operative patients both by breath and conventional methods. The team found significantly elevated concentrations of carbonyl sulfide (COS) in the breath of those subjects with evidence of acute rejection compared to those without. COS, like many sulfides in breath, is thought to be one of the products of L-methionine metabolism, which seems to be enhanced in patients undergoing lung transplant rejection. In a similar study,15 Michael Phillips and his group at the New York Medical Center, US, investigated heart-transplant patients, where again biopsy is the current monitoring method. Heart rejection is accompanied by oxidative stress that degrades membrane polyunsaturated fatty acids, evolving alkanes and methylalkanes, which are excreted in the breath as VOCs. The researchers showed that breath analysis could detect chronic oxidative stress in heart transplant patients with moderate to severe symptoms, pointing to a way to reduce the need for as many biopsies.
Related Links
Journal of Breath Research
This journal is dedicated to all aspects of breath science
References
- T. Wang et al, J. Breath Res., 2008, 2, 1.
- L. Pauling, A. B. Robinson and P. Cary, Proc. Natl Acad. Sci. U. S. A., 1971,68, 2374.
- M. Phillips et al, J. Chromat. B, 1999, 729, 75.
- T. H. Risby and S. F. Solga, Appl. Phys. B, 2006, 85, 421.
- J. K. Schubert et al, Intensive Care Med. , 1998,24, 415.
- M. E. O'Hara et al, Physiological Measurement, 2008, 29, 309.
- J. D. Pleil and A. B. Lindstrom, J. Chromat. B , 1995, 665, 271.
- W. Miekisch and J. K. Schubert, TrAC Trends in Anal. Chem. 2006, 25, 665-673.
- A. Amann, P. Spanel and D.Smith, Mini-Rev. Med. Chem., 2007, 7, 115.
- R. S. Blake, P. S. Monks and A. M. Ellis, Chem. Rev., 2009, 109, 861.
- D. Smith and P. Spanel, Mass Spect. Rev., 2005, 24, 661.
- B. Braden et al, Digestive and Liver Disease, 2007, 39, 795.
- R. S. Blake et al, Anal. Chem., 2004, 76, 3841.
- S. Studer et al, J. Heart and Lung Transpl., 2001, 20, 1158.
- M. Phillips et al, J. Heart and Lung Transplant., 2004, 23, 701.









No comments yet