What real chemists do can be the basis of motivating investigations and learning in school chemistry. A problem faced by a panel of quality control scientists, Which is fresher, supermarket-packed spinach or frozen spinach?, provides the basis of investigative work across Key Stages 3-5.
-
Students extract and analyse chlorophyll molecules and their degradation products from spinach using TLC and HPLC
- Results provide insight into the freshness of green leaves

Analytical chemistry is an integral part of the 2008 chemistry specifications at GCSE and A-level, and, at a basic level of paper chromatography, has been part of Key Stage 3 science for years.1 The new specifications reflect the increasing importance of analytical techniques to chemists and fit in well with 'how science works'.
At GCSE chromatography and retention factors (Rf) have been written into the specifications with a focus on additives in foods, and at A2 some specifications now expect students to interpret retention times and mass spectral data from HPLC-MS to resolve complex mixtures and elucidate the component structures.
This presents chemistry teachers with the difficult task of finding suitable course material that can be taught to students from Key Stages 3-5 which explores the requisite parts of analytical science but which also encourages an investigative approach2 that will satisfy the requirements of 'how science works'. The investigation described here exploits chlorophyll chemistry and is my way of addressing these issues. Entitled Which is fresher, supermarket-packed spinach or frozen spinach?, the investigation can be used at Key Stages 3 and 4, and with modification at Key Stage 5.
Chlorophyll chemistry
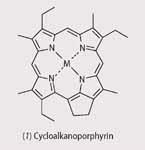
Chlorophyll molecules, the green pigments found in chloroplasts of leaf cells, absorb light and use it to provide energy for photosynthesis.3 Chlorophylls are Mg(II) complexes of cyclic tetrapyrroles similar to porphyrins (1) but differ in that they are saturated between C-17 and 18.
The most abundant chlorophyll in green plants is chlorophyll a (2), though many plants also contain significant amounts of chlorophyll b, which differs from chlorophyll a through the inclusion of an aldehyde group at the C-7 position rather than a methyl group.
Chlorophyll molecules are chemically reactive and decompose once they are removed from the protective environment of the cell. The main degradation products of chlorophyll are grey pigment molecules, the phaeophytins. These molecules differ from the parent chlorophylls in that the central weakly bound Mg atom has been replaced by hydrogen atoms.
Thus any cellular damage caused by ageing of a leaf should show up in an analysis of the chlorophyll molecules and their degradation products,4,5 by using TLC or HPLC, and this would give students a good insight into the freshness of the sample.
This, however, is not the whole story because chlorophyll degradation has a number of pathways, leading to other products, not all of which are detectable by TLC analysis. Chlorophyll allomers form during extraction and analysis of the chlorophylls as a result of autoxidation in organic solvents.
The extent of the formation of chlorophyll allomers depends upon the solvent used in the extraction of the derivatives and the time the chlorophylls are allowed to spend in solution. Alcohols, such as methanol and ethanol, promote the allomerisation of chlorophylls, leading to a series of products (2 (a)-(d))6 and should be avoided during the extraction or analysis.
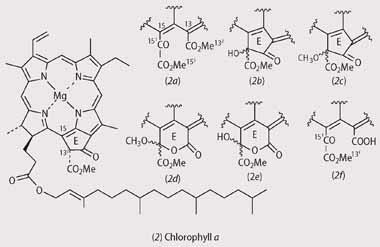
While acetone (propanone) does not promote allomerisation, prolonged exposure of acetone solutions containing water will lead to allomerisation involving 3O2 from the air and/or solvolysis with water molecules to produce a different set of products (2 (b), (e), and (f)).6
Box 1 - Using chlorophylls to assess the freshness of spinach leaves

Plant leaves also contain pigments other than chlorophyll, the most abundant of these are compounds called carotenoids. We can see the wonderfully bright yellows, oranges and reds of carotenoids every autumn in the leaves that fall from trees. We can see them because the green chlorophylls degrade to colourless compounds as autumn progresses to winter.
Chlorophylls are easily degraded and damaged when the leaf cells break down. Looking at the types of chlorophylls and their breakdown products in a leaf can therefore tell us about the health or freshness of a leaf.
The pigments extracted from a plant leaf are in fact a complex mixture of chlorophylls and carotenoids and to separate them we must use chromatography. Paper chromatography can be used to separate simple mixtures such as a mixture of inks from pens, but to separate plant pigments we must use a more efficient method such as thin layer chromatography (TLC).
TLC works in a similar manner to paper chromatography - the pigment moves along the surface of the TLC plate depending upon its partition between the plate surface (stationary phase) and its dissolution in the solute (mobile phase). Each pigment has a different affinity for the plate surface and the mobile phase (partition coefficient) and will therefore move a unique distance along the plate. Developing the TLC plate in the mobile phase for a few minutes lead to the separation of the pigment mixture so that we can assess the freshness of the leaf according to the pigments we can identify that are present on the plate.
The challenge...
The investigation is presented to the students as a series of task statements:
You are a member of a panel of independent quality control scientists, responsible for identifying fresh, nutritious food for local school children. School chefs are looking to include more green vegetables in school meals and have decided that spinach will be a vital part of the exemplar school meal that will be served to celebrity chefs and ministers at the launch of a new range of school meals.
The chefs want to include the freshest ingredients possible, but there is some disagreement as to whether supermarket-packaged spinach or frozen spinach is the freshest. The freshest spinach will have the most intact chlorophyll a and the least phaeophytin a. You and your team must prepare and test the spinach samples for their chlorophyll content according to your experimental brief. Finally, you must answer the question, Which is fresher, supermarket-packed spinach or frozen spinach?, in a report which includes the evidence from your scientific observations and experiments.
Be prepared to present your own work and to assess the work of others so that your results are in agreement.
Box 2 - Experimental procedure
During this experiment you should wear goggles and follow all laboratory safety procedures. Work quickly to obtain the best results.
- Collect a pre-cut 3 × 7 cm TLC plate (silica gel 60 Å, Whatman, UK) and using a pencil and a ruler, carefully draw a horizontal thin line 1.5 cm above the base of the plate. Be careful not to gorge the surface of the plate with your pencil and do not touch the surface of the plate.
- Collect a single large spinach leaf and grind with 20 drops of acetone in a pestle and mortar.
- Pipette the acetone extract into a watch glass which has been secured to the lab bench by using Blu-Tack on the underside of the dish.
- Use a hair dryer to remove all water from the extract. Repeat if necessary by re-dissolving the extract in a few drops of acetone.
- When the extract is dry, add three drops of acetone to re-dissolve the pigments.
- Use a micro-pipette to transfer the pigment solution onto the TLC plate. Repeat until the dot is dark green.
- Pipette the elution solvent (9:1 petroleum ether/ acetone) to a height of 1 cm in the elution tube. Carefully slot the TLC plate into the tube, wrap the tube in foil and leave to develop.
- After about four minutes, check the TLC plate, remove and immediately mark off the solvent front with a pencil. Circle any observable spots with pencil.
- Measure the distance run by the solvent in millimetres and each pigment spot that you observe. Measurements should be made from the centre of the original spot to the front of each pigment spot.
- Calculate a unique Rf value for each pigment. (Rf = distance run by pigment divided by distance run by solvent.)
- Repeat the process for the thawed frozen spinach.
- Draw a suitable table of results. Identify each pigment in both samples. Use your data to assess the freshness of each sample based on the amount of observable phaeophytin a. Write these results into a report which includes an introduction to your experimental task, your results and your conclusions. Remember to say why you made your conclusions and what results led you to them.
Key Stages 3 and 4
Classes of Key Stage 3 and 4 students are given the task along with a brief containing some background on how chlorophyll chemistry can be used to assess the freshness of the spinach, and an experimental procedure. Teachers must at this point complete a detailed risk assessment, taking into account the risk of handling flammable solvents. After reading the brief and discussing the problem as a group, the students do the experiment.
Results
The results of TLC analysis of the chlorophyll extracts were clear enough for the students to base a qualitative conclusion as to the freshness of the samples.
- Supermarket-bought spinach exhibited four spots upon TLC analysis using a 9:1 (v/v) mixture of light petroleum ether (B.P. 40-60°C) and acetone as the elution solvent. At the top of the plate, near the solvent front, was a strong yellow spot assigned as β-carotene; below this was a blue/green spot, assigned as chlorophyll a; below this was a dark-green spot, assigned as chlorophyll b; and last was an additional yellow spot, assigned as a xanthophyll.
- Frozen spinach exhibited five spots upon TLC analysis. At the top of the plate was a strong yellow spot, assigned as β-carotene, below this was a dark-grey spot, assigned as phaeophytin a; below this was a blue/green spot, assigned chlorophyll a; below this was a dark-green spot, assigned as chlorophyll b; and last was a yellow spot, assigned to xanthophyll.
Supermarket-bought spinach was found to be the freshest of the samples ,owing to the lack of a dark-grey TLC spot for phaeophytin a. Student-calculated Rf values for chlorophyll a varied from 0.41 to 0.50 and for phaeophytin a from 0.63 to 0.69, respectively. (Literature Rf values of 0.59 for chlorophyll a and 0.81 for phaeophytin a have been reported for a 3:1:1 petroleum ether, acetone, chloroform solvent system.)7
A few common problems
The students had few difficulties in understanding the problem and obtaining a TLC from the crude chlorophyll extract. However, during the experiment and the subsequent write-up of the results they did share some common problems, which affected the accuracy of their results:
- chlorophylls are subject to a number of chemical reactions which involve cleavage of the macrocyclic ring if left in contact with light for long periods. These breakdown products will eventually lead to a decolourisation of the chlorophyll solution under strong light and lead to a build up of chlorophyll degradation products, which will affect the results of the TLC analysis of the chlorophylls within the spinach samples. This problem can be avoided by using aluminium foil as a wrapper, to keep out light from solutions containing chlorophyll;
- the hydrogen atom at the C-13 position of the chlorophyll molecule is subject to allomerisation with 3O2 in air.2,5 The products of this reaction all include an oxygen atom bound to C-13, and some include solvent molecules in their structure. These products, allomers, can go undetected by TLC, merging into the chlorophyll a spot. This problem can be solved by completing the practical within a single or double period, so that the chlorophyll solutions are not in contact with air for long. If chlorophyll solutions must be saved they can be stored in a freezer and used as soon as is practicable;
- once filtered the crude chlorophyll extract is loaded onto the TLC plate by using a micro-pipette. The micro-pipette will only take up a very small amount of the chlorophyll solution, so students will get better results if they repeat the loading of the chlorophyll solution onto the TLC plate at least five times. This makes minor constituents of the mixture, such as phaeophytin a, easier to observe with the naked eye;
- the students did not appreciate how quickly the solvent would evaporate from the TLC plate once they had taken it out of the elution tank and exposed it to the air. Some students took too long before they marked the solvent front with pencil and therefore created inaccuracies in their measurement of the Rf values for chlorophyll a and phaeophytin a.
Extending the investigation to Key Stage 5
A useful extension activity for Key Stage 5 students is the analysis, by TLC and HPLC-MS, of the chlorophylls contained in a wet acetone extract of supermarket-bought spinach that has been stored in the refrigerator for a week after initial extraction. The basis of this challenge is for students to test for any transformation of the chlorophylls during this period.
Results
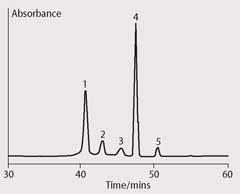
TLC of the extract revealed a product distribution identical to that observed in the original investigation. Analysis of the extract by HPLC, according to a published method,8 revealed a product distribution of five peaks with differing retention times (tR) (Fig 1). Identification of the chlorophyll derivatives was based upon their retention time and the parent ion mass of the chlorophyll derivatives after APCI-HPLC-MS (atmospheric pressure chemical ionisation-high performance liquid chromatography-mass spectrometry) analysis.9
The first peak was assigned as Mg-purpurin-7 monomethyl ester (2 (f), [M+H]+ 925), the second peak as 132(S,R)-hydroxychlorophyll a (2 (b), [M+H]+ 909), the third peak as 151(S,R)-hydroxylactonechlorophyll a (2 (e), [M+H]+ 925), the fourth as 132(R)-chlorophyll a (2, [M+H]+ 893) and the fifth as the lesser chlorophyll diasteromer 132(S)-chlorophyll a (2, [M+H]+ 893).
The HPLC analysis illustrated the complexity of the original mixture, the HPLC separating five chlorophyll transformation products from the single spot observed in TLC.
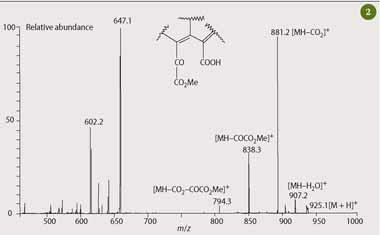
As a further extension to this activity, students could be challenged to confirm the assignment of the peaks observed in the HPLC chromatogram, which are based on parent ion masses and retention time. This can be done by assigning the fragment ions in the mass spectrum of the main chlorophyll transformation product observed in the HPLC chromatogram (Fig 1, peak 1) of Mg-purpurin-7 monomethyl ester. The mass spectrum of this transformation product exhibits diagnostic fragment ions from the altered ring-E portion of the chlorophyll molecule which is sufficient to allow students to elucidate and confirm the structural assignment given the main mass losses from the pseudomolecular ion [M+H]+ as shown in Fig 2.
The final frontier...
Once the students had appreciated the complexity of chlorophyll chemistry and the analytical challenge in separating their derivatives effectively, I set a final challenge to a small team of KS5 students to produce a sample of pure chlorophyll a from spinach.10 This pure sample could then be used as a standard in the school laboratory to check the identity of the chlorophyll a spot observed during the TLC analysis of the chlorophylls extracted from spinach.
Box 3 - Extracting pure chlorophyll a

Rinse spinach (200 g) with deionised water and dry between paper towels. Homogenise the spinach in a blender with acetone (500 ml) for three minutes. Filter the resulting solution through cotton wool to remove debris. Collect the resulting solution and add 1,4-dioxane (70 ml), followed by enough distilled water (around 30 ml) to precipitate microcrystalline chlorophyll. Decant the supernatant, dissolve the chlorophyll precipitate in acetone (200 ml) and divide between four large centrifuge bottles. Centrifuge the chlorophyll solutions at 2000×g for five minutes to remove any remaining cellular debris. Combine the supernatants and then remove acetone by using a rotary evaporator. Purify the solution by large-scale column chromatography.12
Purification:
Pack a large glass chromatography column (7 cm id × 50 cm) with icing sugar by using a water pump and wash the column with one bed volume of hexane. Dissolve a portion of the crude chlorophyll extract in diethyl ether (ethoxyethane, 3 ml) and carefully pipett onto the top of the column. Develop the column initially with portions of hexane (200 ml) and then with portions of 0.1-0.5 per cent 2-propanol in hexane until the chlorophyll fractions have eluted.
The elution order of the pigment fractions is: carotenoids (yellow), phaeophytin a (grey), chlorophyll a epimer (green), chlorophyll a (blue/green) and chlorophyll b (olive green). Combine the pure eluents and remove the solvents in vacuo.
My students obtained a yield of ~5 mg of chlorophyll a. To test the efficiency of the chromatographic separation and therefore the purity of the sample, they analysed a small portion of chlorophyll a in acetone by infusion into an APCI-equipped mass spectrometer. They followed a literature method involving the post column addition of methanoic acid to improve the analytical sensitivity of the mass spectrometer.11
Chlorophylls do not protonate well under the conditions of APCI, owing to the central Mg atom, so it is better to convert them to phaeophytins because these molecules accept a proton more readily in the APCI interface. Analytical sensitivity is improved by an order of magnitude.
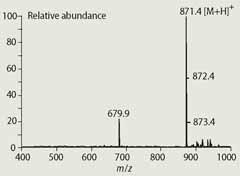
The resulting mass spectrum (Fig 3) exhibits a strong [M+H]+ parent ion at m/z 871, which is consistent with its assignment as pure chlorophyll a that has been converted to phaeophytin a during the analysis. The ion observed at m/z 679.9 is an impurity, possibly a carotenoid. The APCI-HPLC-MS analysis therefore gave the students a rapid method for assessing the purity of their chlorophyll a.
Pure samples of the pigments within spinach obtained this way are useful standards to identify the chlorophyll derivatives within the original student brief if they are spotted on the TLC plate along with the original spinach extracts.
And finally...
By giving the students a real problem to investigate encourages them to take part in the activity thoughtfully. This approach also creates an opportunity for students to see that chemistry is relevant to the community and the school as a whole, which mirrors the objectives of 'how science works'. Furthermore, writing the results in the form of a report encourages literacy and scientifically articulate writing that can be peer reviewed, mirroring a real scientific process.
Dr Stuart Walker teaches chemistry at Ridgewood School, Barnsley Road, Scawsby, Doncaster DN5 7UB.
Acknowledgements
My thanks to Planet Science, the Engineering and Physical Sciences Research Council (EPSRC) and the University of York for support and facilities.
References
- K. Roberts, Educ. Chem., 2007, 44 (6), 162.
- S. Walker, Educ. Chem., 2005, 42 (6), 168.
- H. Scheer in Chlorophylls, H. Scheer (ed), pp3-30. Boca Raton, US: CRC, 1991.
- G. R. Seely in The chlorophylls, L. P. Vernon and G. R. Seely (eds), p67. New York: Academic, 1966.
- P. H. Hynninen in Chlorophylls, H. Scheer (ed), pp145-209. Boca Raton, US: CRC, 1991.
- S. Walker, PhD thesis, pp56-94. University of York, UK, 2004.
- C. Reiss in Experiments in plant physiology, C. Reiss (ed). Englewood Cliffs, US: Prentice Hall, 1994.
- R. L. Airs, J. E. Atkinson and B. J. Keely, J. Chrom. A, 2001, 917, 167.
- C. Jie, S. Walker and B. J. Keely, Rapid Commun. Mass Spectrom., 2002, 16, 473-479.
- K. Iriyama, N. Ogura and A.Takamiya, J. Biochem., 1974, 76, 901.
- W. A. Svec in Chlorophylls, H. Scheer (ed), pp89-102. Boca Raton, US: CRC, 1991.
- R. L. Airs and B. J. Keely, Rapid Commun. Mass Spectrom., 2000, 14, 125.









No comments yet