Green chemistry is a maturing discipline. But the subject still holds big challenges that the next generation of chemists must tackle, as Josh Howgego reports

Chemistry has a dark side. Behind every ounce of innovative plastic, invaluable drug or fresh research insight, there can be a mountain of solvent, electricity, heavy metals and their associated emissions.
Whether the pollution produced outweighs the benefits these new products and ideas bring is a deep and seldom considered question. But for more than 20 years now there have been chemists who want to make that darkness a good deal greener. They are working to make sure that the impacts of large-scale industrial chemistry are minimised. Their profession, known as ‘green chemistry’, is now fairly mature – though as we’ll see there are still plenty of difficult problems left for chemistry’s next generation to solve.
Pregabalin goes green
A good way to understand green chemistry is to look at how the industrial synthesis of pregabalin has evolved over the years. This drug molecule, developed by pharmaceutical firm Pfizer, is used to treat partial seizures, chronic pain and anxiety disorders. In 2013 Pfizer sold more pregabalin than anything else: £2.7 billion worth of it.
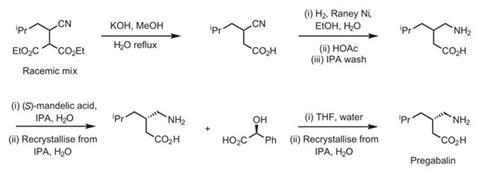
The firm published their first synthetic route to the molecule in 1997. This involved five basic steps, all of which used organic solvents such as methanol and isopropyl alcohol (scheme 1). (There are two reasons why such solvents aren’t green. First, they originate from crude oil, a non-renewable resource. And second they are generally incinerated after they’ve become contaminated, releasing carbon dioxide into the atmosphere.) But the real problem with the synthesis from a green chemistry point of view was the separation of the two enantiomers of the final product. The synthesis used a classical method of separation, a recrystallisation with (S)-mandelic acid as a co-crystallising agent. But after the crystallisation the mandelic acid was thrown away – and since a 1:1 ratio of acid:product was required large volumes of waste were generated.
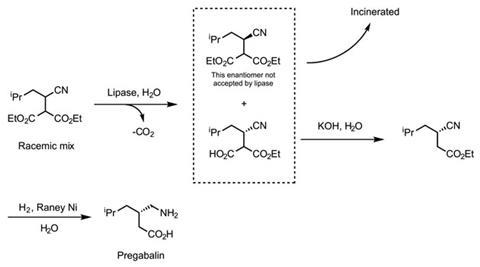
Yet the principles of green chemistry had been gaining ground in the 1990s, and Pfizer soon introduced a second generation of the manufacturing process to pregabalin (scheme 2). The key discovery Pfizer made was that it was possible to use an enzyme, a type of lipase, to convert the diester starting material to the corresponding acid – and that the enzyme only accepted the required diastereomeric form of the diester. This brought two big advantages. First, the reaction could be run in water (such reactions must be conducted in water to avoid denaturation of the enzyme). Water causes far fewer problems than the organic solvent used in the first generation process because it is environmentally benign so doesn’t need to be incinerated after use, as organic solvents do. Instead it can usually be purified and recycled or released into a sewage system. Second, the enzymatic separation of the diastereomers means that the need for the co-crystallisation agent used previously – and the associated waste – was avoided.
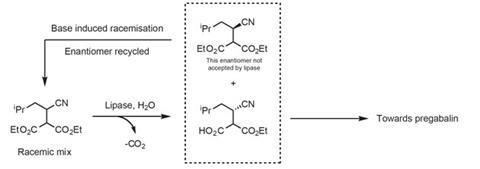
The second generation process was a big improvement on the first. But it still left half of the starting material, the unwanted diastereomer of the racemic diester, unused as it wasn’t accepted by the lipase. This both wasted Pfizer’s money and the incineration of the unused material produced carbon dioxide. But the company eventually discovered a trump card. It found that adding a base to the unwanted diastereomer would induce a racemisation – that is, the leftover diastereomer would be converted into a 1:1 mixture of diastereomers. This effectively transformed a useless compound back into the starting material, meaning Pfizer could recycle what was previously wasted (scheme 3). Though this could only be repeated two or three times before the process became uneconomical.
Pfizer’s own calculations indicate that the introduction of the enzymatic step will mean that the production of more than 3 million tonnes of carbon dioxide is avoided between 2007 and 2020, compared to using the original manufacturing route. That’s equivalent to a cube of gas with sides about four times the height of the Eiffel tower in Paris, France.
How green is ‘green’?
There are many assumptions behind Pfizer’s figures on the carbon dioxide savings. Calculating a carbon footprint is a fraught business because the figures you come out with depend on what is considered part of the chemical process and what isn’t. We should be wary of this. But still, it’s obvious that removing the co-crystallisation agents and swapping organic solvents for water mean less waste and carbon dioxide being produced, and in that sense the process is ‘greener’. But how much greener, exactly?
This is a vital question, particularly for chemists considering several synthetic routes that could be implemented on an industrial scale. The routes to pregabalin discussed above were chosen from several alternatives. So it’s important for chemists to be able to quantify the ‘greenness’ of a synthesis, so they know which one to opt for.
Unfortunately greenness doesn’t come in buckets or bars. Instead, chemists have had to agree standard ways of assessing syntheses so they can be compared with one another. Take the E-factor (environmental factor) for example. This was one of the first green chemistry metrics. It was introduced in the 1980s by Roger Sheldon, who now works at the University of Delft in the Netherlands. An E-factor is a useful, if crude, metric that counts how much mass is wasted during a chemical process. It can be calculated using the simple formula:
E-factor = mass of waste / mass of product
In the pregabalin example, the E-factor went from 86 in the first process, to 17 in the second and just 10 in the current one.1 (A higher number indicates a larger quantity of waste being produced). This means that less waste is generated per unit of product.
This gives a useful indication of how much greener the successive processes are. But E-factors do not take account of environmental hazards such as the toxicity of the waste or the amount of energy the process requires. These other factors are important. We can see how, for example, a process conducted at room temperature may well more green overall than one that needs heating to 70°C – even though the latter could have a lower E-factor.
No single green chemistry metric is ever enough to get a full picture of a process’ greenness, says David Constable, director of the American Chemical Society’s Green Chemistry Institute in Washington DC, US. There are a whole heap of other measures you can use, says David, who has edited a book on green chemistry metrics.2 The important thing to decide is which question you want to answer; then you can select a collection of metrics that will give you a realistic insight from several angles at once. ‘You have to take a multi-metric approach, and each of those metrics will tell you different things,’ he says.
One of the most important other metrics, says David, is the lifecycle assessment. This looks at what industrial chemists call ‘impact factors’, he explains. These include things like release of carbon dioxide, nitrogen compounds in water courses (known as eutrophication), photochemical ozone creation potential, acidification, and so on. ‘There can be up to 12 or so different categories,’ says David, and assessing a process’ impacts in each area gives chemists an idea of where they could direct their efforts for maximum reduction of negative environmental impact.
‘The other approach a lot of people use is to identify “materials of concern”,’ explains David. ‘So you look at all the reactants, solvents, filter aids and so on that go into your process and you try to do an assessment of the hazard and the risk profile based on those materials.’

Within this many companies focus their efforts on solvents, simply because they make up the majority of the total mass used in a process – usually 50 or 60% is organic solvents and 20 or 30% is water, according to David. Because of this pharmaceutical companies now publish solvent selection guides based on each material’s health and environmental credentials.3
But David says the industry still has some way to go. ‘One thing that in my opinion is not really looked at enough is energy,’ he says. And he also argues that sometimes metrics can be inappropriately simplified.
‘What lifecycle assessments have been reduced to in most places is carbon footprinting; which to me is anathema. Just looking at the carbon footprint is a problem because you do get into situations, particularly where you’re looking at the use phase of the drug, where other impacts become really important. The classic example I use is how asthma inhalers were formulated with CFCs [chlorofluorocarbons] as the propellant and you had a huge problem with ozone depletion in the use phase – not in the manufacture. They switched the propellent from CFCs to HCFCs [hydrochlorofluorocarbons], but that just switched the problem from ozone depletion to global warming. The way to get around that is to use dry powder inhalers, although the very young and the very old have problems using them.’
Although metrics are complicated, there are some interventions chemists can make to ensure processes are greener without worrying too much. Replacing solvents with water is one such idea. David says that more and more reactions that people would never have thought would be possible in water are becoming tenable. Another trick chemists can play is to use micelles. These are essentially soap-like droplets dispersed within an aqueous bulk solvent. Reactions that require a relatively non-polar organic solvent can sometimes be conducted in an emulsion (a mixture of water and oily solvent) like this, and the overall effect is to reduce the amount of organic solvent used. He also says the emerging discipline of synthetic biology – where cells’ machinery is re-programmed to produce specific, useful compounds – deserves more research attention because it too is generally conducted in water. It also can be used to make very specific chemical transformations such as asymmetric syntheses without the use of less desirable organometallic catalysts.
Regulation concerns
Whether it is a boost to the fundamental research in synthetic biology or targeted tweaks to existing industrial process, green chemistry is built on research. And that costs money.
Peter Dunn, a green chemistry expert at Pfizer, has written that unfortunately there is a ‘major financial disincentive’ for pharmaceutical companies to do this research. The reason, he says, is that new chemical processes have to be approved by regulators before they can be used to sell drugs, and there are hugely variable approval times between regulators serving different countries.
Green chemistry is built on research. And that costs money.
David explains that getting approval for a new process involves companies submitting outlines of their new processes to the regulator for a given country or region along with a huge amount of data that proves that the final drug compound has not changed from what has been previously approved. Of course, the drug’s chemical structure won’t have changed, but there is potential that changes in catalysts and solvents might introduce changes to the drug’s impurity profile or crystal structure. Pharmaceutical firms need to prove to their regulators that this hasn’t happened.
Peter conducted research in 2013 that showed approval could happen in some countries within a month, and others could take more than 26 months.1 This means that the company is forced to either delay setting up the second generation process until all nations have approved it or run two different processes to make the same product at once. Clearly both options are financially problematic.
It might sound surprising that these drugs aren’t regulated on a global scale. That would surely avoid these problems. But David explains that concerns over certain sub-populations having different reactions to drugs are what drives opposition to this idea.
‘Japan, for instance, is a market where you have to do a completely different set of studies to elsewhere,’ he explains. ‘That has to do with the genetic profile of the people in Japan. Because that is a very [genetically] homogeneous culture, they require studies to be done in Japan to rule out any problems that might arise from that difference in genetic make up.’
Yet ‘most people in the pharmaceutical industry would argue that if the appropriate trials have been done and they satisfy the EMA [European medicines Agency] or the US FDA [Food and Drug Administration], then there should be a way to get international agreement about what’s going to satisfy people,’ says David.
By the time the current generation of chemistry students are ready to move into industry, it will be roughly two decades since green chemistry ideas started being taken seriously. Pressure on the environment looks set only to increase, and so the discipline will only become more important. Perhaps it will be up to this generation to answer the biggest challenges green chemistry faces: how to measure its impacts and how to ensure the changes it brings are safe without consuming research budgets.
Josh Howgego is a science journalist based in London, UK
References
- P J Dunn, Green Chem., 2013, 15, 3099 (DOI: 10.1039/c3gc41376d)
- A Lapkin and D Constable, Green chemistry metrics: measuring and monitoring sustainable processes. Wiley-Blackwell, 2008
- P Dunn, Chem. Soc. Rev., 2012, 41, 1452 (DOI: 10.1039/c1cs15041c)









No comments yet