Help 14–16 learners link redox reactions to electron gain and loss

Redox reactions are all around us. Some, such as photosynthesis, respiration and combustion, are essential to life. Others, like rusting, are mildly annoying. Oxidation makes sense to students when expressed in terms of oxygen. Oxidation is gain of oxygen and reduction is loss of oxygen. This definition can be traced back to Antoine Lavoisier’s experiments to disprove the phlogiston theory in the late 1700s.
1. Make examples explicit
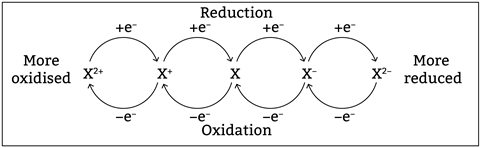
To understand a broader range of reactions, students need to work with definitions of oxidation and reduction in terms of electrons. This is an abstract concept, so doesn’t naturally capture the interest of teenagers. It also quickly ramps up in difficulty from simple definitions to redox half-equations, which require a solid understanding of core concepts of atomic structure and bonding.
OIL (oxidation is loss) RIG (reduction is gain) is a classic mnemonic that really helps with this key idea. Reinforce this definition by providing lots of different examples of equations and asking students to identify which elements are being oxidised and which reduced. Start with half-equations which make the electrons explicit. Later, you can add stretch and challenge by getting students to separate out half-equations from an overall equation and link different areas of learning.
2. Break down equations
Pace your topic coverage according to your class. The initial ideas are simple but there is no point proceeding into writing half-equations if your class struggles to balance normal symbol equations that don’t have charges. Spend time building confidence in identifying the species oxidised and reduced from given equations and build up to students writing their own. Look at past assessment questions to see what kinds of species examiners set so you can scaffold practice accordingly.
When moving onto half-equations, reduce the fear. Half-equations can look intimidating. Breaking the process down into a set of rules can provide the structure needed for success. The process of balancing half-equations can be summarised into two simple steps, which must be completed in order. Start by balancing the atoms. Then balance the charges by adding in electrons.
3. Practise, practise, practise
Provide lots of examples. It doesn’t matter if they’re not the standard ones from exam papers, it’s an opportunity for the students to see these two steps work in all the circumstances they may experience.
Check with your assessment body about where they allow electrons in half-equations. Many examining bodies allow electrons to be either subtracted or added. Ideally students should only add electrons in half-equations, but some lower achieving students do find it more intuitive to subtract electrons.
Using iron, adding electrons to balance the charge follows the accepted convention: Fe → Fe2+ + 2e-
However, taking away electrons may seem more logical to some students: Fe – 2e- → Fe2+
4. Model mastery
Help students navigate the three corners of Johnstone’s triangle to develop mastery. Incorporate well-planned demonstrations, such as the combustion of iron wool, into lessons to capture interest. Students observe the phenomena (macroscopic), represent it with equations (symbolic) and consider what is happening on a particle scale (microscopic).
Help students navigate the three corners of Johnstone’s triangle to develop mastery. Incorporate well-planned demonstrations, such as the combustion of iron wool (rsc.li/45r5qdP), into lessons to capture interest. Students observe the phenomena (macroscopic), represent it with equations (symbolic) and consider what is happening on a particle scale (microscopic).
Reassure students that redox in terms of oxygen and redox in terms of electrons is the same thing. Students can worry when a new definition comes along that they feel conflicts with ideas they are confident in. You can prove the definitions agree using familiar examples, such as prior learning on the extraction of metals or the burning of iron wool demo you may have shown. The balanced symbol equation for the reaction between iron and oxygen is 4Fe + 3O2 → 2Fe2O3. Separating this out into the two elements and balancing the charges with electrons gives you Fe → Fe3+ + 3e- and O2 + 4e- → 2O2-
Some students will need this explanation so that they can move on in their thinking, whereas others will accept the electron focused definition without question.
Kristy Turner













1 Reader's comment