Get started
Watch a demonstration of this experiment and download the technician notes from the Education in Chemistry website: rsc.li/49hvlFV
The random motion of particles explains many spontaneous phenomena. Although diffusive processes in gases can be seen fairly quickly, similar demonstrations in liquids can be frustratingly slow. A carefully placed crystal of potassium permanganate (KMnO4) will take most of a lesson to dissolve and spread across the base of a beaker.
In this variation of the Traube cell reaction, a bubble of blue liquid first floats and then sinks as water diffuses out into the surrounding yellow liquid, which demonstrates in seconds the diffusion of water molecules. You can explore diffusion and density with 11–14 learners using this demonstration. You can also use it with 16–18 learners to develop their understanding of transition metal chemistry and hone their standard solution preparations.
The random motion of particles explains many spontaneous phenomena. Although diffusive processes in gases can be seen fairly quickly, similar demonstrations in liquids can be frustratingly slow. A carefully placed crystal of potassium permanganate (KMnO4) will take most of a lesson to dissolve and spread across the base of a beaker.
In this variation of the Traube cell reaction, a bubble of blue liquid first floats and then sinks as water diffuses out into the surrounding yellow liquid, which demonstrates in seconds the diffusion of water molecules. You can explore diffusion and density with 11–14 learners using this demonstration. You can also use it with 16–18 learners to develop their understanding of transition metal chemistry and hone their standard solution preparations.
Kit
- 10 cm3 of a 0.4265 M copper(II) sulfate (CuSO4) solution
- 40 cm3 of a 0.2808 M potassium hexacyanoferrate(II) (K4Fe(CN)6) solution
- Boiling tube or large test tube (20 × 150 mm)
- Clamp and stand, or 250 cm3 cell culture flask
- Dropping pipette (see tips)
Preparation
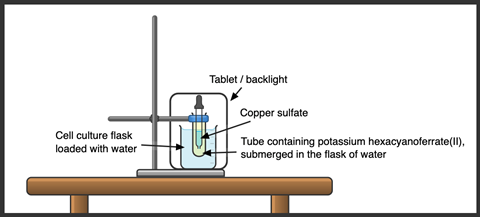
The copper(II) sulfate and potassium hexacyanoferrate(II) solutions, when made up as detailed in the kit list, should result in the densities of two solutions being around 0.2% apart, so it’s essential to be careful with the preparation of the solutions. You should make up the stock accurately in at least a 100 cm3 volumetric flask, which can then be used for multiple demonstrations.
Record the mass of a 100 cm3 volumetric flask before carefully weighing out 10.65 g of copper(II) sulfate pentahydrate (CuSO4.5H2O) and transferring it into the volumetric flask. Add 50–75 cm3 of deionised water to the flask and swirl to fully dissolve the copper(II) sulfate. Then slowly add deionised water to the flask up to the line, mix thoroughly and record the final mass. Repeat the process in another weighed 100 cm3 volumetric flask with 11.86 g of potassium hexacyanoferrate(II) (K4[Fe(CN)6].3H2O). Be aware, potassium hexacyanoferrate(II) is yellow and must not be confused with the orange potassium hexacyanoferrate(III).
Clamp the boiling tube and pour in 40 cm3 of the yellow potassium hexacyanoferrate(II) solution leaving space at the top for displacement by the pipette. Decant roughly 10 cm3 of the blue copper(II) sulfate solution into a small beaker to avoid contaminating stock with the pipette.
Health, safety and disposal
- Wear eye protection.
- Avoid exposing potassium hexacyanoferrate(II) to any heat sources. CLEAPSS members should consult HC079.
- Copper(II) sulfate is harmful if swallowed and can cause serious eye damage. Avoid contact with skin. CLEAPSS members should consult HC027c.
- For disposal, filter off precipitate and dilute solutions with plenty of water. Dispose of diluted solutions down a foul-water drain.
Health, safety and disposal
- Wear eye protection.
- Avoid exposing potassium hexacyanoferrate(II) to any heat sources. CLEAPSS members should consult HC079 (bit.ly/49b46N8).
- Copper(II) sulfate is harmful if swallowed and can cause serious eye damage. Avoid contact with skin. CLEAPSS members should consult HC027c (bit.ly/3UKCcDh).
- For disposal, filter off precipitate and dilute solutions with plenty of water. Dispose of diluted solutions down a foul-water drain.
In front of the class
Fill the pipette with enough blue copper(II) sulfate solution to deliver a pea-sized droplet (see tips). Insert the dropper carefully, minimising disturbance to the yellow solution, until the tip is 1–2 cm above the base of the tube. Inject the droplet and remove the pipette quickly (this helps to initiate the upward motion if your solutions are too close in density). A red-brown membrane forms around the droplet and it will begin to rise. After a few moments at the surface, the droplet falls to the base of the tube where, over the next two minutes it will visibly shrink and the membrane will shrivel.
You can repeat the process, until the density of the surrounding yellow solution matches that of the blue solution being introduced.
Tips
- Always double-check the densities of the copper sulfate and potassium hexacyanoferrate solutions after making them up – if the copper solution sinks then the demonstration will fail.
- For larger classes, use a visualiser or webcam so students can see the details. Clamp the tube in an oblong container filled with water to reduce the lensing effect from the water in the tube and greatly improve the visibility of the droplets on a screen. A 250 cm3 cell culture flask combined with a medium (20 × 150 mm) test tube is ideal for this, and you can place the apparatus in front of a white backdrop or screen to backlight the solutions.
- Although an ordinary teat pipette can be used for this demonstration, a Pasteur pipette attached to a small syringe with some silicone tubing requires less dexterity. You can find tips in the accompanying video.
Tips
- Always double-check the copper sulfate and potassium hexacyanoferrate solutions after making them up – if the copper solution sinks then the demonstration will fail.
- For larger classes, a visualiser or webcam will be needed to see the details. Clamp the tube in an oblong container filled with water to reduce the lensing effect from the water in the tube and greatly improve the visibility of the droplets on a screen. A 250 cm3 cell culture flask combined with a medium (20 × 150 mm) test tube is ideal for this, and you can place the apparatus in front of a white backdrop or screen to backlight the solutions.
- Although an ordinary teat pipette can be used for this demonstration, a Pasteur pipette attached to a small syringe with some silicone tubing requires less dexterity. You can find tips in the accompanying video (rsc.li/3OJxF0c).
Teaching goal
After adding the copper(II) sulfate to the potassium hexacyanoferrate(II) solution, a colloidal solid membrane of copper hexacyanoferrate(II) will form around the droplet. The balanced equation for this reaction is:
2 Cu2+ (aq) + Fe(CN)64– (aq) → Cu2Fe(CN)6 (s)
Calculate the respective densities by dividing the mass of the liquids in the flasks by 100. The blue solution has a density close to 1.062 g cm–3 whereas the yellow solution should be close to 1.065 g cm–3. This small difference in density causes the blue droplet to rise slowly.
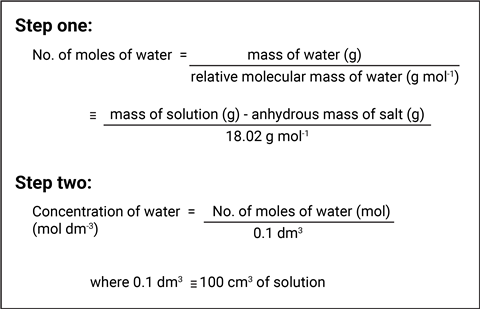
Students should be aware of the process of diffusion – the movement of particles from areas of high to low concentration through random motion. If a membrane separates particles, this can prevent some substances from diffusing as readily. In this case, water diffuses into the yellow potassium hexacyanoferrate(II) solution, which, even though it has a lower concentration of solute, it has a higher concentration of water. This may seem counterintuitive to students, so remind them that you are interested in the concentration of the water in each solution. The water particles are lighter than hexaaquacopper(II) ions so, once the water has diffused out, the density inside the droplet increases and it sinks. You can calculate the concentration of the water in the solutions to show that the concentration of water is higher in the blue solution.
In this example, 6.81 g of copper sulfate and 10.34 g of potassium hexacyanoferrate(II) were dissolved, which means that the concentrations for the water are roughly 55.2 mol dm–3 and 53.4 mol dm–3 in the blue and yellow solutions, respectively. This explains why water would diffuse out of the droplet.
Teaching goal
After adding the copper(II) sulfate to the potassium hexacyanoferrate(II) solution, a colloidal solid ‘membrane’ of copper hexacyanoferrate(II) will form around the droplet. The balanced equation for this reaction is:
2 Cu2+ (aq) + Fe(CN)64– (aq) → Cu2Fe(CN)6 (s)
Densities can be calculated by dividing the mass of the liquids in the flasks by 100. The blue solution has a density close to 1.062 g cm–3 whereas the yellow solution should be close to 1.065 g cm–3. This small difference in density causes the blue droplet to rise slowly.
Students should be aware of the process of diffusion, the movement of particles from areas of high to low concentration through random motion. If a membrane separates particles, this can prevent some substances from diffusing as readily. In our case, water diffuses into the yellow potassium hexacyanoferrate(II) solution, which, even though it has a lower concentration of solute, it has a higher concentration of water. This may seem counterintuitive to students, so remind them that you are interested in the concentration of the water in each solution. The water particles are lighter than hexaaquacopper(II) ions so, once the water has diffused out, the density inside the droplet increases and it sinks.
You can calculate the concentration of the water in the solutions to show that the concentration of water is higher in the blue solution. This explains why water would diffuse out of the droplet.
More resources
- Explore the properties of water and develop your 11–14 students’ method-writing, planning and problem-solving skills with this ice-making practical.
- Expand on the topic of concentration in your 14–16 classes with this practical video showing how concentration affects rates of reaction.
- Reinforce your 16–18 learners’ understanding with these questions on transition metal chemistry.
- For more inspiring demonstrations, check out the Exhibition chemistry section of our website.
Downloads
Go with the flow technician notes
Editable handout | Word, Size 0.47 mbGo with the flow technician notes
Handout | PDF, Size 0.18 mb

























No comments yet