The UK recently faced a CO2 shortage. But why did it happen when there is too much in the atmosphere?

What’s the first thing that comes into your head when you hear the words ‘carbon dioxide’? Climate change? Respiration? Fizzy drinks? The fact is, carbon dioxide (CO2) is one of the most important gases in our lives and recently we had both too much of it and also not enough.
In the everyday news cycle, CO2 is portrayed as the bad boy of atmospheric gases. Its ever-increasing presence in the atmosphere contributes to global warming and climate change, which threatens our very existence on the planet. But recently another CO2-related problem has reared its ugly head: there wasn’t enough of it in the UK’s chemical supply chain to satisfy the myriad uses of this essential feedstock in manufacturing, healthcare and food production.
In your class
Discuss some examples of carbon dioxide usage in industry from this article. Learners should be able to recall the processes by which carbon dioxide moves into or out of the atmosphere. Ask learners why it might be difficult to extract carbon dioxide from the atmosphere for use in industrial applications. In the case of a CO2 shortage, could learners prioritise which industries should have access to the CO2 supply?
Being thermodynamically stable, heavier than air and with no liquid phase at atmospheric pressure, CO2 is one of the most useful industrial gases we have, says Mark Lorch, head of chemistry, biochemistry and chemical engineering at the University of Hull. ‘When added to beverages, it gives them their fizz. Trap it in high pressure bubbles in sweets and you get popping candy. Compress it in a cylinder and you have a fire extinguisher. Freeze it and you produce dry ice, which is used to keep medical materials, including Covid vaccines, chilled during transport,’ he explains.
‘The microbial organisms that cause food to perish need oxygen to survive, so packaging salad leaves with CO₂, not oxygen, keeps them fresh. Meanwhile, in the meat industry, high concentrations of the gas are used to replace oxygen in the air animals breathe, rendering them unconscious before they are slaughtered,’ Mark continues.
In fact, it’s in the food industry that the shortage of CO2 was felt most keenly (it’s also used in greenhouses to promote plant growth and to decaffeinate coffee), and threatened stocks on supermarket shelves.
What’s happening?
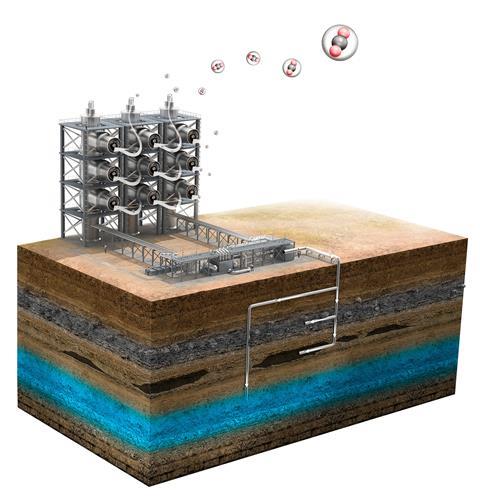
To fully understand why there was a shortage of carbon dioxide, we need to look at how it’s made. The main industrial source is the manufacture of ammonia-based fertiliser, of which CO2 is a by-product. Ammonia is made using the Haber process, combining nitrogen and hydrogen under pressure to form NH3.
To give an idea of the importance of this reaction, Mark says that around 80% of the nitrogen in our bodies has come via the Haber process, making this single chemical reaction ‘probably the most important factor in the population explosion of the past 100 years’.
Of the ingredients needed, N2 is very abundant (it’s 78% of the atmosphere we breathe) but hydrogen gas is not, so must be synthesised. This is most commonly achieved via methane steam reforming, which involves taking natural gas and heating it in the presence of water to about 1000˚C. The end products are hydrogen gas and carbon dioxide.
Download this
Worksheet, for age range 11–14
A worksheet to establish prior knowledge and then assess understanding of the carbon cycle. Use these questions in conjunction with our carbon cycle game and lesson plan.
Download the student worksheet as MS Word or pdf and the teacher notes as MS Word or pdf.
A worksheet to assess prior knowledge and understanding of the carbon cycle
Download the student worksheet and teacher notes from the Education in Chemistry website: rsc.li/3FtvGpI
The hydrogen gas goes on to be used in the production of ammonia, while the CO2 is either captured and sold as a commodity or vented into the atmosphere as waste. However, not all fertiliser plants are geared up for CO2 capture, reveals Mark. ‘Unfortunately, the amount of CO₂ produced by the fertiliser industry far exceeds the quantity needed by other industries, so most fertiliser plants don’t bother capturing it,’ he says.
So, what has all this got to do with the recent shortage of CO2? The problem is the natural gas starting material, or rather the cost of it. During 2021 the price of gas has increased from just under 50p per therm to over £2.50 per therm. This is because of factors such as a cold winter in 2020, calm weather reducing the amount of electricity generated by wind and the economy waking up again after the Covid-19 lockdown.
In fact, so huge is the price of natural gas that it recently became uneconomical for some fertiliser plants to operate, so they shut down.
All our eggs in one basket
The fact that the price of natural gas can influence the availability of a completely different commodity further down the supply chain gives you an indication of the UK’s dependence on this one single source of CO2 production. In fact, just two fertiliser plants in the north of England supply over 60% of the food industry’s CO2 requirements and it’s these two plants (owned and run by US operator CF Industries) that temporarily shut down.
The situation is the same across Europe, where there is also a natural gas shortage, but the UK is worse hit because we don’t have as much gas storage. We rely on a just-in-time model instead, buying gas when it’s needed on the wholesale market.
‘There are technologies that can capture CO2 from the air but since the source of CO2 is very dilute, it is relatively expensive’
Just how control of most of the UK’s fertiliser and CO2 production has come to end up in the hands of a single US-owned company is a question that politicians will have to answer, but chemists can do something to prevent this problem reoccurring, by coming up with new ways to produce CO2.
Something in the air
On hearing about the CO2 shortage, most people will instantly think: ‘Hang on a minute! If we have too much CO2 in the atmosphere, why can’t we just take some of that and use it in the food, health and agriculture industries?’ And that’s a great question.
Currently, we emit around 40 billion tons of CO2 into the atmosphere every year. These emissions are the primary driver of climate change. However, as the CO2 is spread over such a vast area, it is very dilute in the air. Just 0.04% is CO2, compared to 78% nitrogen and 21% oxygen. This means any device that captures CO2 from the air is going to have to work really hard to scavenge enough for our industrial needs. Using current technology, that means the process won’t be economically feasible.
However, as the price of clean electricity falls, carbon-capture technology is becoming a more realistic prospect, says Greg Mutch, Royal Academy of Engineering research fellow at Newcastle University. ‘This gets very tricky, very quickly,’ he warns. ‘There are technologies that can capture CO2 from the air – known as direct air capture – but since the source of CO2 is very dilute, it is relatively expensive to capture CO2 from the air, compared with fertiliser production or using more traditional carbon capture and storage (CCS) technology on power plant flue gas.’
A big part of the decarbonisation agenda is to produce hydrogen cleanly
Greg explains that direct air capture plants rely on contact sorbents – ‘materials with chemical functionality that reacts with CO2 but leaves other elements in the air alone. Once the CO2 sorbent is regenerated, a pure stream of the gas can be recovered and used industrially. Although for the purposes of climate change mitigation, it should be stored underground in geological sites.
‘Compared to traditional CCS technology, which captures from sources containing about 5–15% CO2, direct air capture costs more to operate and requires more energy to drive the process,’ he says.
More resources
- Examine the role that carbon dioxide played in the supermarket’s empty shelves with this starter slide for 14–16 year olds.
- Connect students’ learning about energy and the carbon cycle to the UN’s Sustainable development goals with this activity for 14–16 year olds.
- Ensure your students have a firm understanding of the chemistry behind climate change with this CPD article on how to teach the carbon cycle at 11–14.
- Link to careers with this job profile of an atmospheric chemist studying pollutants; find more inspiration with our Fixing the future video.
- Examine the role that carbon dioxide played in the supermarket’s empty shelves with a starter slide for 14-16 year olds: rsc.li/3nCI5Bs
- Connect students’ learning about energy and the carbon cycle to the UN’s Sustainable development goals with this discussion activity for 14–16 year olds: rsc.li/3nBN1qo
- Ensure your students have a firm understanding of the chemistry behind climate change with this CPD article on how to teach the carbon cycle at 11–14: rsc.li/3nMssaZ
It seems that direct air capture is a technology in its infancy. If it’s going to be useful as an industrial route to CO2, as well as capturing it for green purposes, this will certainly be in the long term. So, what can be done in the short term to safeguard our salad and get the bubbles back in our lager? Mark says there are alternatives that will help in a pinch: ‘The most obvious is nitrogen gas, which can be used in the same way as CO₂ to preserve food or stun animals. Likewise, because nothing burns in nitrogen, it can be used to suppress fires – just like CO₂ fire extinguishers.’
Additionally, the government is currently in crisis talks with CF Industries, with the aim of subsidising the production of fertiliser at the company’s two plants and restarting CO2 production. But again, this seems to be a short-term fix for a problem that has occurred before (in 2018, when it was blamed on a high demand for lager during the football World Cup), and will surely happen again.
Mark says the current fertiliser route to CO2 is ripe for an overhaul, since it relies on a fossil fuel starting material and a 100 year-old chemical process, plus it produces a greenhouse gas by-product. ‘A big part of the decarbonisation agenda is to produce hydrogen cleanly for use in fertilisers and fuel. One of the simplest ways to achieve this is via the electrolysis of water, using clean sources of electricity,’ he says. ‘So, as carbon capture technologies develop, we may see CO2 extracted from the air for use in industrial processes.’
How long this takes to develop will be down to the resourcefulness of researchers at the cutting edge of environmental science, but if the current CO2 shortage has taught us anything, it’s that the complex chemical supply chains upon which we rely for so much of our food and drink are increasingly fragile.
Article by Ian Farrell, a freelance journalist and photographer, based in Cambridge. Resource by Holly Walsh, a teacher and research lead












No comments yet