Use these classroom ideas, tips and resources to effectively teach this challenging topic
Molecules are one of the key particles in chemistry. An understanding of molecules helps us explain much of the material and natural world around us.
At 14–16, students need to understand three main points about molecules.
- Covalently bonded substances can form small molecules.
- Weak intermolecular forces exist between molecules.
- The bigger the molecule, the stronger the intermolecular forces.
These points may seem simple enough, but students often struggle to visualise what molecules are and how they are different from giant structures. We also introduce the idea of intermolecular forces often without any explanation on how they form.
Clearing up misconceptions
In my experience these are the most common misconceptions that students have about molecules.
- Every substance with a formula exists as molecules.
- Covalent bonds are broken when a molecular substance changes state.
- Intermolecular forces exist in all substances.
When I first introduce molecules to students, I emphasise that all substances exist as molecules or giant structures, in order to counteract these misconceptions. I focus on the discrete nature of molecules and explain the difference in meaning of formulas (for molecules) and empirical formulas (for giant structures).
You can also counteract misconceptions about intermolecular forces by looking beyond the way they’re often taught at this level. This is helpful because the 14–16 model lacks certain ideas that can’t be explained, such as:
- How intermolecular forces form.
- Bonding is not just purely ionic or covalent – some molecules show some ionic character.
- Molecules with the same molecular mass have different boiling points.
- Why molecules do or do not dissolve in water.
In my experience the most common misconceptions that students have about molecules are: every substance with a formula exists as molecules; covalent bonds are broken when a molecular substance changes state; and intermolecular forces exist in all substances.
When I first introduce molecules to students, I emphasise that all substances exist as molecules or giant structures, in order to counteract these misconceptions. I focus on the discrete nature of molecules and explain the difference in meaning of formulas (for molecules) and empirical formulas (for giant structures).
You can also counteract misconceptions about intermolecular forces by looking beyond the way they’re often taught at this level. This is helpful because the 14–16 model lacks certain ideas that can’t be explained, such as:
- How intermolecular forces form.
- Bonding is not just purely ionic or covalent – some molecules show some ionic character.
- Molecules with the same molecular mass have different boiling points.
- Why molecules do or do not dissolve in water.
Temporary dipoles
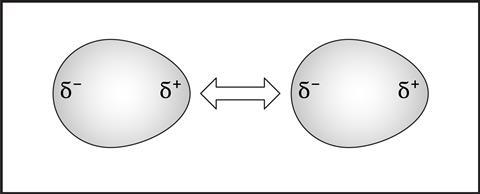
Intermolecular forces is a catchall term for several different types of attraction between particles. We discuss London forces at 14–16, which can be described as transient, induced dipole–dipole interactions. They form because small fluctuations in the electron charge cloud of a molecule cause small temporary areas of negative or positive charge to form. These are temporary dipoles, also called transient or instantaneous dipoles. A temporary dipole in one molecule can induce a dipole in a neighbouring molecule and the two dipoles can attract each other.
The bigger the molecule, the more dipoles will form, and so the stronger the attractive force. This explains why halogens have increasing melting points down the group. The forces can be large enough to make a substance a solid at room temperature, such as iodine. It also explains the idea that the bigger the alkane, the higher the boiling points and viscosity.
When discussing intermolecular forces with 14–16 students, it’s important to reinforce the idea that all bonding is electrostatic in nature and that London forces are just the weakest example. All molecules have these forces but, at 16–18, students are introduced to other types of intermolecular forces. This can help explain properties like anomalously high melting points of some molecules and differences in solubility in water.
Permanent dipoles and polar molecules
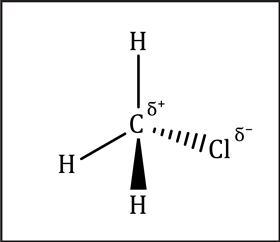
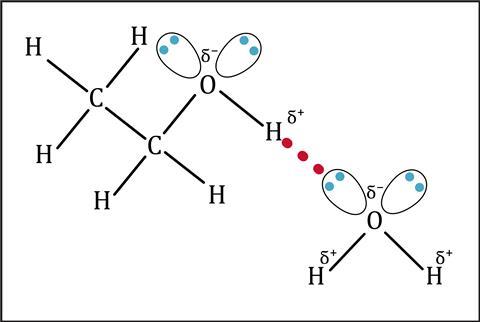
In a covalent bond the electrons are not always shared equally. All atoms of elements attract the bonding electrons more or less strongly, in a sort of electron tug of war. We call this property electronegativity – the stronger the pull, the higher the electronegativity. Elements such as fluorine, oxygen, nitrogen and chlorine have high electronegativities. Atoms of these elements in molecules therefore tend to bear a small negative charge, with other bonded atoms bearing a small positive charge. This charge separation is permanent, so we call this a permanent dipole.
Permanent dipoles in one molecule can attract the dipole in a neighbouring molecule. These permanent dipole–dipole interactions are stronger than London forces, and occur in addition to London forces. Substances with permanent dipoles tend to have higher boiling points than substances with only London forces between the molecules.
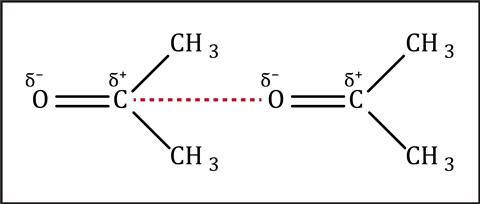
Molecules with permanent dipoles are often called polar molecules, whereas ones that only form temporary dipoles are called nonpolar. The polar nature of substances can affect both the physical and chemical properties of a substance. The existence of polar molecules is a useful insight for students into the continuum of bonding between ionic and covalent.
Resources for your classroom
- Use Starter for 10 activities 3.1.3 and 3.1.4 to help identify different types of bonding and to help students to correct poor use of vocabulary.
- Learn more about students’ misconceptions of molecules and bonding with this summary of education research.
Resources for your classroom
- Use Starter for 10 activities on identifying different types of bonding to help students to correct poor use of vocabulary: rsc.li/3HI5USv
- Learn more about students’ misconceptions of molecules and bonding with this summary of education research: rsc.li/3wEKu2j
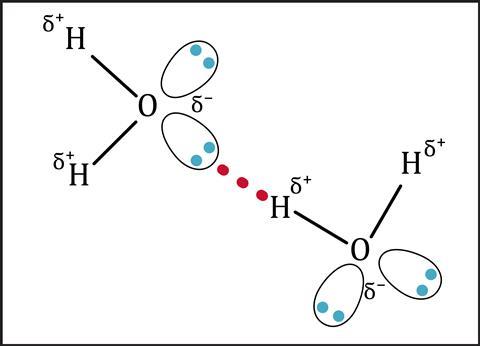
Hydrogen bonding
When atoms of the three most electronegative elements (nitrogen, oxygen and fluorine) bond to hydrogen atoms, a particularly strong dipole forms. The lone pair of electrons on these electronegative atoms are attracted to the positive hydrogen atoms in a neighbouring molecule. The small size of hydrogen atoms allows a close approach of the two atoms, which makes the force of attraction stronger. This is hydrogen bonding of the intermolecular forces. Compounds that exhibit hydrogen bonding, like water and ammonia, have anomalously high boiling points compared to those that do not exhibit this type of bonding.
Comparing boiling points
Here are three examples of the boiling points of substances with similar relative molecular masses:
-
Propane: C3H8 (Mr = 44) has only London forces, and has a boiling point of -42°C
- Ethanal: C2H4O (Mr = 44) has permanent dipole–dipole attractions, and has a boiling point of 20°C
- Ethanol: C2H6O (Mr = 46) has hydrogen bonding, and has a boiling point of 78°C
Molecules with significant hydrogen bonding, such as ammonia and ethanol, can dissolve in water because they can form hydrogen bonds with the water molecules. Nonpolar molecules such as hexane will not be able to form hydrogen bonds with water and so will be immiscible in water.
Take-home points
- Be careful to correctly use the word molecule from first teaching at 11–14.
- At 11–14, introduce the idea that all substances (except noble gases) are either molecules or giant structures, and clearly differentiate between molecules and giant structures.
- Reinforce the idea that the formula of a molecular substance is the actual number of atoms of each element, whereas with a giant structure the formula is only a ratio of elements (called the empirical formula).
- Do not use the term relative molecular mass for ionic substances; use the term relative formula mass.
- When first introducing intermolecular forces, be clear and state there is more than one type. Reinforce the idea they are all electrostatic in nature, like all types of bonding. Use this article to explore this idea: Covalent bonding made easy.
- As a class, look at poorly written student descriptions of molecules and intermolecular forces and discuss how they could be improved.
Take-home points
- Be careful to correctly use the word molecule from first teaching at 11–14.
- At 11–14, introduce the idea that all substances (except noble gases) are either molecules or giant structures, and clearly differentiate between molecules and giant structures.
- Reinforce the idea that the formula of a molecular substance is the actual number of atoms of each element, whereas with a giant structure the formula is only a ratio of elements (called empirical formula).
- Do not use the term relative molecular mass for ionic substances; use the term relative formula mass.
- When first introducing intermolecular forces, be clear and state there is more than one type. Reinforce the idea they are all electrostatic in nature, like all types of bonding. This article on covalent bonding explores this idea: rsc.li/3Rf3DkY.
- As a class, look at poorly written student descriptions of molecules and intermolecular forces and discuss how they could be improved.














2 readers' comments