Keep students’ spirits high when learning about alcohols
Alcohols are in many everyday products. For example, ethanol is in alcoholic drinks, such as beer, wine and spirits. It can also be used as a biofuel for vehicles. Most UK petrol stations sell E10 fuel that is a blend of 10% bioethanol and 90% petrol; it’s the bioethanol that lowers the carbon footprint of the fuel. Some internal combustion engines can use pure anhydrous ethanol, but the engines have to be modified or designed for that purpose, for example those in racing cars. Alcohols are also used in many other ways, such as solvents for perfumes and cosmetics, or as feedstock to produce other chemicals, such as esters.

What students need to know
The alcohols are a homologous series of organic molecules with the general formula CnH2n+1OH. They contain the functional group –OH. Like hydrocarbons, alcohols are named by using the prefixes meth-, eth-, prop- and but-, and with the suffix -ol. So the first four alcohols are methanol, ethanol, propanol and butanol.
Physical properties, such as boiling point, density and viscosity, gradually increases with the increasing number of C atoms present in the molecule, whereas solubility in water gradually decreases.
Alcohols, like the hydrocarbons, burn easily in air but it is the hydroxyl group, –OH, that is responsible for their typical chemical reactions. These include:
- oxidation to produce a carboxylic acid.
- reactions with sodium to produce, for example, sodium ethoxide.
- esterification reactions: alcohol + carboxylic acid → ester + water.
Misconceptions and what you need to know
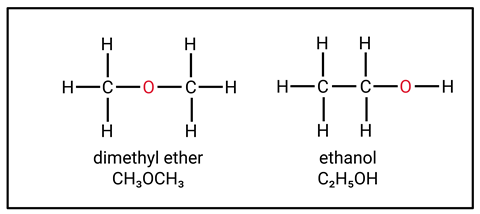
Students can develop some misconceptions based on how we teach organic chemistry at 14–16 that can then cause problems post-16. For example, when writing chemical formulas be sure to use the structural or displayed formula rather than the molecular formula, which may represent more than one molecule (figure 1). Both ethanol and dimethyl ether share the same molecular formula, C2H6O, but are very different chemicals that belong to different homologous series.
When drawing the displayed formula of butanol, students may come across four different structural formulas (figure 2). These are isomers, compounds with the same molecular formula but with the atoms bonded in different orders. While students don’t need to know the names or structures at 14–16, it can be helpful to know that such arrangements are possible. These differences are important at post-16 when students go on to learn how the arrangement of atoms in a molecule can influence a reaction mechanism and lead to different products.
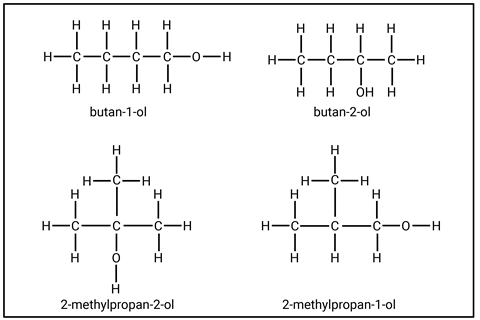
At post-16 students learn how to classify alcohols as primary, secondary or tertiary and go on to look at how their reactions differ. For example, the products of an oxidation reaction depend on the isomer.
- Butan-1-ol (a primary alcohol) → butanal → butanoic acid
- Butan-2-ol (a secondary alcohol) → butanone
- 2-methylpropan-2-ol (a tertiary alcohol) cannot be oxidised.
Suggestions for your teaching
The whoosh bottle is an eye-catching and exciting demonstration that you can use to introduce the alcohols. Start with propanol and ask learners to draw the displayed formula. After the demonstration, pass around the bottle, so that learners can feel the temperature increase from the exothermic reaction. Pour out the water produced, having previously emphasised the importance of emptying the bottle of any unvaporised fuel. You get a significant volume of liquid. Ask learners to write a chemical equation, and suggest how you can test the products. Provided you have enough whoosh bottles, move on to ethanol and methanol and ask learners to predict any differences they expect to see.
This demonstration clearly illustrates that chemicals within a homologous series behave in similar ways. It lays the foundations for further study at post-16 when they will investigate the enthalpy of combustion using calorimetry to collect experimental data and compare the results with that calculated from bond enthalpies.
Encourage learners to make their own molecular models with Molymods and draw out the displayed formulas. This activity helps to address misconceptions around atom connectivity (specifically the C–O bond) at post-16. Highlighting the hydroxyl group in the alcohol helps learners distinguish it from a hydroxide ion (OH–), which can cause confusion.
Use diagnostic probes to explore students’ understanding of molecular structure. The worksheet on propane and propanol from the BEST project investigates whether learners can recognise that it is the structures of organic molecules that give rise to differences in the forces of attraction between molecules, which explains the differences in melting and boiling point of organic compounds. A good understanding of intermolecular forces at post-16 is key to a good understanding of organic chemistry.
Because alcohols are flammable, they must be handled with care. At post-16, learners need to confidently handle these substances and use a range of techniques to synthesise both an organic liquid and solid. Therefore, it is important to develop good practical skills at 14–16 by making good use of the opportunities for class practical work. Oxidising primary alcohols gives a good clear result and fills your room with the sweet smell of esters. These practicals also provide another opportunity to discuss the wide-ranging opportunities for chemists in industry.
- The whoosh bottle (rsc.li/47Kse9N) is an eye-catching and exciting demonstration that you can use to introduce the alcohols. Start with propanol and ask learners to draw the displayed formula. After the demonstration, pass around the bottle, so that learners can feel the temperature increase from the exothermic reaction. Pour out the water produced, having previously emphasised the importance of emptying the bottle of any unvaporised fuel. You get a significant volume of liquid. Ask learners to write a chemical equation, and suggest how you can test the products. Provided you have enough whoosh bottles, move on to ethanol and methanol and ask learners to predict any differences they expect to see. This demonstration clearly illustrates that chemicals within a homologous series behave in similar ways and lays the foundations for further study at post-16 when they will investigate the enthalpy of combustion using calorimetry (rsc.li/4owJGps) to collect experimental data and compare the results with that calculated from bond enthalpies.
- Encourage learners to make their own molecular models with Molymods and draw out the displayed formulas. This activity helps to address misconceptions around atom connectivity (specifically the C–O bond) at post-16. Highlighting the hydroxyl group in the alcohol helps learners distinguish it from a hydroxide ion (OH–), which can cause confusion.
- Use diagnostic probes to explore students’ understanding of molecular structure. The worksheet on propane and propanol from the BEST project (rsc.li/3JBQGlT) investigates whether learners can recognise that it is the structures of organic molecules that give rise to differences in the forces of attraction between molecules, which explains the differences in melting and boiling point of organic compounds. A good understanding of intermolecular forces at post-16 is key to a good understanding of organic chemistry.
- Because alcohols are flammable, they must be handled with care. At post-16, learners need to confidently handle these substances and use a range of techniques to synthesise both an organic liquid and solid. Therefore, it is important to develop good practical skills at 14–16 by making good use of the opportunities for class practical work. Oxidising primary alcohols gives a good clear result and fills your room with the sweet smell of esters (rsc.li/47Awo5m). These practicals also provide another opportunity to discuss the wide-ranging opportunities for chemists in industry.
Resources for your classroom
- Use the comprehensive alcohols worksheet to provide contexts for your learners (whoosh bottle for the higher level and cooking for the foundation level) and to assess their understanding.
- Check learners’ end of topic knowledge with the alkanes and alcohols worksheet. Questions are structured in the form of a reactions map, which is a useful revision tool.
- Show a video to promote the career of forensic scientist, Joni and discuss how she uses chemistry in her job.
- Identify learning gaps and misconceptions with the Review my learning worksheets.
- Use the comprehensive alcohols worksheet to provide contexts for your learners (whoosh bottle for the higher level and cooking for the foundation level) and to assess their understanding: rsc.li/3LAzyNS
- Check learners’ end of topic knowledge with the alkanes and alcohols worksheet: rsc.li/3LuBEUJ. Questions are structured in the form of a reactions map, which is a useful revision tool.
- Show a video to promote the career of a forensic scientist and discuss how Joni uses chemistry in her job: rsc.li/47LOKzc
- Identify learning gaps and misconceptions with the Review my learning worksheets: rsc.li/4qNwNZX
Dorothy Warren is an independent science education consultant based in the UK














1 Reader's comment