Use real life examples to help students get to grips with the properties of ceramics, polymers and composites
A quick look around any school building reveals a huge variety of different materials. Different materials have different properties, and each material is carefully chosen to match the needs of the job it is performing.

Ceramics, for example, are strong, hard and rigid, making them suitable for uses ranging from dainty tea sets to toilets. They are formed by heating or baking an inorganic material (such as a metal oxide) to a high temperature in an oven. They are strong and hard, but brittle. The word ceramic comes from the Greek word keramikos, meaning ‘potter’s clay’. While pottery is an obvious example, others include bricks and, when coated in a glaze, sinks and toilets are also made from ceramic.
Polymers, meanwhile, are also strong and tough but – unlike ceramics – tend to be flexible. A polymer is a large molecule formed from a chemical reaction that joins many smaller, carbon-based molecules together. Polymers also tend to be chemically unreactive, solids at room temperature, plastic (malleable and able to be moulded into shape) and electrical insulators. Their uses range from plastic bags to clothing or water pipes.
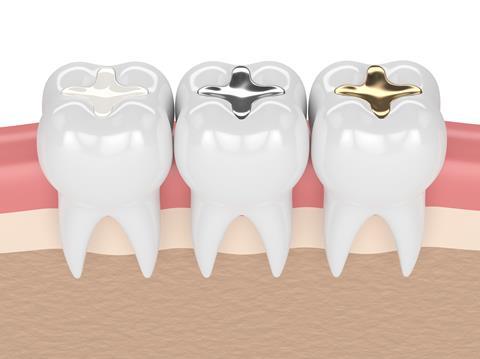
Lots of the materials around us are composite. A composite material is made by combining two or more other materials – often ones with very different properties – to create a more useful material. The two materials work together to give the combination of properties. However, within the composite you can easily tell the different materials apart as they do not dissolve or blend into each other. Steel-reinforced concrete is a good example. This is made by pouring concrete around a steel cable mesh and has the natural strength properties of both the concrete and the steel. White teeth fillings are also a composite material, made of a mixture of plastic and fine glass particles to give them their required enamel-mimicking properties.
What students need to know
- The properties of ceramics, polymers and composites.
- Some examples of ceramics, polymers and composites. Their properties are easier for students to remember when linked to everyday examples.
- Students do not need to understand why these material classes have these properties – this tends to be explored at 14–16.
Ideas for your classroom
Molymod polymers
Ask students to create ethene or another monomer using Molymods. Discuss its properties and explain that it is a small molecule. Next, one student breaks a ‘bond’ between the two carbons in their model and forms a new bond with a neighbouring student’s monomer. This dimer should then form another bond with another student’s monomer. This continues around the classroom until all the monomers are linked to form a single, much larger molecule – a polymer. Next, discuss the relative sizes of the starting material compared to the polymer. Ask how the sizes relate to the properties. An example lesson plan and slides are available in the downloads.
Download this
A lesson presentation and activities (as MS Powerpoint or pdf) with teacher notes (as MS Word or pdf) on the properties of polymers and how they are formed.
A lesson presentation and activities with teacher notes on the properties of polymers to use with your students from the Education in Chemistry website: LINK
PVA polymer slime
Making slime is a great demonstration of how properties can change when new bonds form. It isn’t an addition reaction happening here, rather a change in properties due to cross linking between existing polymer chains, making this a useful demonstration or class practical if you have a class ready to progress to key stage 4 content. Ask students to compare the properties of starting materials and the product to determine what might have happened.
Olympic composites
This reading comprehension contains many examples of composites used in the Olympic Games. There is a related resource for making a composite pot from fabric and a flour and water paste.
Making concrete

Concrete is a composite containing a number of materials such as cement, gravel, sand and water that combine to give it its properties. Ask students to develop a hypothesis about how the amount of cement in a concrete mix will affect the final strength, then use this resource to test their hypothesis experimentally. Significant figures or decimal places can be introduced when recording the results if appropriate. This also provides a good opportunity to share results between groups and to revise calculating the mean.
Concrete is a composite containing a number of materials such as cement, gravel, sand and water that combine to give it its properties. Ask students to develop a hypothesis about how the amount of cement in a concrete mix will affect the final strength, then use this resource (LINK) to test their hypothesis experimentally. Significant figures or decimal places can be introduced when recording the results if appropriate. This also provides a good opportunity to share results between groups and to revise calculating the mean.
Common misconceptions
Students can sometimes mix up composites with elements, compounds and even alloys. Care must also be taken when students asks whether an alloy is a composite or not. An alloy always contains at least one metal, whereas composites do not necessarily. Furthermore, composites are always heterogeneous and often contain reinforcement materials.
At this level, it may be beneficial to avoid discussion of intermolecular forces in polymers.
Formative assessment
Use regular quizzes – retrieval practice – to support learning of the properties of ceramics, polymers and composites.
A true or false quiz can quickly determine how well a class has understood a concept. They can be expanded by asking students to explain why they chose a particular answer.
The Quizlet app offers different ways of quizzing your students; try this one that I made on the topic.
The Quizlet app offers different ways of quizzing your students; try this one that I made on the topic: LINK
Progression to 14–16
At 14–16, students learn more about polymers. As chemical bonding is introduced, the links between the properties of materials and their bonding is made clearer. For example, the bonding in addition polymers is taught – the double bond in the alkene monomer breaks to form a bond with another monomer. Condensation polymers may also be discussed. Formulations are taught, which links into what a composite is – a formulation is a mixture designed for a specific purpose.
Take home points
- Ceramics, polymers and composites have desirable properties that make them useful materials.
- Be clear about the difference between a composite and an alloy.
- Remember that teaching 11–14 can be frustrating at times as it feels like you cannot give your students a ‘proper’ answer without going into too much detail. Use lots of examples and make the topic applicable to their lives.
- Further down the line it is possible for your students to understand the ‘why’ questions in more detail.












No comments yet