Help your 14–16 students get to grips with all forms of carbon
In terms of mass, carbon is the second most abundant element in the human body and the fourth most abundant element in the universe. It forms the basic building blocks of life including proteins, carbohydrates and fats. Carbon bonds in several different ways and the different structures that it forms have different properties. It can bond to form one of the hardest substances (diamond) as well as very soft substances (such as graphite) and many more besides.
This interesting topic introduces students to the structure, bonding and therefore properties of the following, all formed from just carbon (known as the allotropes of carbon):
- diamond
- graphene
- graphite
- fullerenes (including buckminsterfullerene)
Before you can explain the properties of each of the different allotropes of carbon, students need to have a secure understanding of atomic structure and the structure and bonding of simple covalent molecules.
Before you can explain the properties of each of the different allotropes of carbon, students need to have a secure understanding of atomic structure and the structure and bonding of simple covalent molecules (rsc.li/4h1NiN8).
What students need to know
Some common themes help students to recall the properties of the different allotropes of carbon and help to avoid common misconceptions.
Melting points
In all allotropes of carbon, the carbon atoms are held together by strong covalent bonds that form one, very large, or macro, molecule. These covalent bonds require a lot of energy to break.
Therefore, the different allotropes all have high melting and boiling points. For example, the melting point of diamond is 4500°C, graphite and graphene’s melting point is around 3650°C.
Electrical conductivity
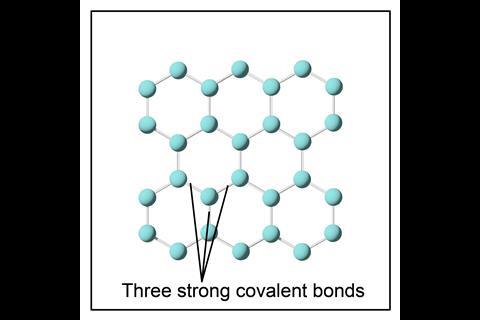
When considering whether an allotrope of carbon can conduct electricity, it is important to understand the bonding, and particularly the electrons. In diamond, each carbon atom is bonded to four other carbon atoms, so all the electrons are involved in bonding.
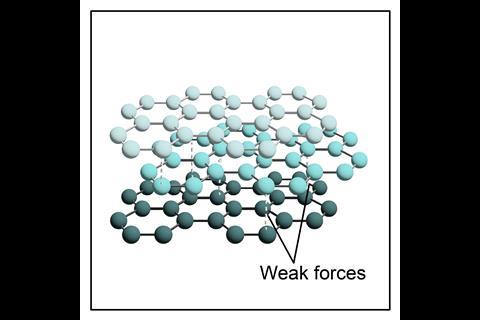
In graphite and graphene, each carbon atom is only bonded to three other carbon atoms, which means each carbon atom has one electron that becomes delocalised. Delocalised electrons are free to move throughout the structure and can carry an electrical charge, so graphite and graphene both conduct electricity.
Why not bring this point to life in your classrooms by creating graphite circuits with a soft pencil lead.
Why not bring this point to life in your classrooms by creating graphite circuits (bit.ly/4eebgSJ) with a soft pencil lead.
Other allotropes of carbon
Graphene – a single layer of graphite formed from carbon atoms bonded to three other carbon atoms in hexagonal rings. Due to the strong C–C bonds in graphene, it is also incredibly strong, lightweight and flexible.
The delocalised electrons in graphene mean it is great conductor of electricity and it is believed that using graphene in batteries will increase the lifespan of lithium batteries in our phones as well as reduce the charging time down to minutes rather than hours.
The delocalised electrons in graphene mean it is great conductor of electricity and it is believed that using graphene in batteries will increase the lifespan of lithium batteries in our phones as well as reduce the charging time down (bit.ly/4fdc0Z5) to minutes rather than hours.
Although theorised in the 1940s, graphene wasn’t successfully isolated until 2004 at the University of Manchester by Andre Greim and Kostya Novosolev, who won the 2010 Nobel prize in physics. They used adhesive tape to peel layers of graphite apart. This works well on a small scale, but larger scale production of graphene is much more difficult and research continues in this area.
Although theorised in the 1940s, graphene wasn’t successfully isolated until 2004 at the University of Manchester by Andre Greim and Kostya Novosolev, who won the 2010 Nobel prize in physics (bit.ly/3YwrcLk). They used adhesive tape to peel layers of graphite apart. This works well on a small scale, but larger scale production of graphene is much more difficult and research continues in this area.
Use Graphene: future applications to help students with retrieval of key properties for each of the different allotropes.
Use Graphene: future applications (rsc.li/400nD10) to help students with retrieval of key properties for each of the different allotropes.
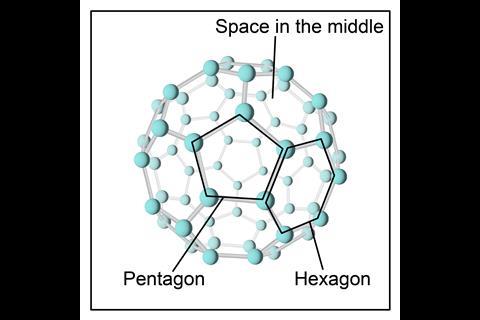
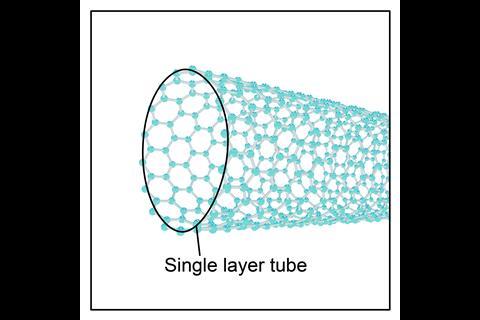
Fullerenes – molecular allotropes of carbon that form hollow shapes. Examples of fullerenes for students aged 14–16 include spheres (like C60) and tubes (known as carbon nanotubes).
Buckminsterfullerene (C60) is a sphere in which each carbon atom has three covalent bonds to neighbouring carbon atoms. Its recognisable shape – alternating pentagons and hexagons – is like a football. Because of weak intermolecular forces, Buckminsterfullerene has a low melting point.
Lots of other examples of fullerenes exist, however buckminsterfullerene (C60) is the most common naturally occurring one and is found in soot as well as in space. Fullerenes such as gold nanoparticles have been used for improving drug delivery.
Carbon nanotubes are made from single or multiple layers of graphene that are rolled to form a tube. Due to the three strong covalent bonds between each carbon atom they are strong and conduct electricity well. As well as this, carbon nanotubes have high tensile strength and have a high length to diameter ratio. Applications of nanotubes include electronics and nanotechnology.
Suggestions for teaching
To help students understand why graphite has delocalised electrons I like to ask them to hold up four fingers on one hand to represent the four outer shell electrons and ask the class, if it forms three covalent bonds, how many are left? This helps students visually see that each carbon will have one electron left and reinforces the idea of delocalisation as these electrons can carry the charge through the structure.
I like to tell my students about my Dad talking about friends who have never left their home town – ‘He is a right local lad’. This is followed by ‘If local means they don’t move, what must delocalised mean?’ and students seem to remember this. Other alternatives like ‘is the local shop close or far away?’ help students to link the idea of ‘local’ meaning ‘close by’ and therefore delocalised to being far away or moving.
It is worth noting that at 14–16 we teach the structure of graphite as hexagonal layers, whereas at 16–18 we teach the term trigonal planar (describing the arrangement around the carbon atom).
It is important to discuss common misconceptions and use well-designed diagnostic questions to identify areas your students are struggling with
Common misconceptions
The topic of structure and bonding is one that students often find difficult. One common misconception is that students write that the reason small covalent substances have low boiling points is because they contain ‘weak bonds’ rather than ‘weak intermolecular forces’. In relation to allotropes of carbon this misconception may stem from students believing that diamond is a single molecule and not a macromolecule formed from many carbon atoms bonded throughout with four strong covalent bonds each. It is important to discuss common misconceptions and use well-designed diagnostic questions to identify areas your students are struggling with.
An example I use to explain that when a small covalent molecule boils, it is the weak intermolecular forces that are overcome rather than the bonds, is to ask my class what happens when we boil a kettle. When the water in a kettle boils, what comes out the top? Steam, which is water in gas form. I then ask if this steam is still H2O. They will say yes and then you can state that if it is still H2O, no chemical bonds (O–H bonds specifically) have been broken. Instead, it is the forces between the molecules that have been broken.
More resources
See all of our supporting resources for allotropes of carbon at a glance in our structure and bonding resources package for learners aged 14–16.
- Use allotropes of carbon structure strips to help students develop their literacy skills in science.
- Identify learning gaps and misconceptions with differentiated worksheets for students.
- Encourage learners to think about carbon allotropes at different conceptual levels with Johnstone’s triangle worksheets, then deepen their understanding with Developing understanding worksheets.
- Enrich your students’ learning by sharing the story of Harry Kroto, a co-discoverer of buckminsterfullerene, C60
- Display the allotropes of carbon poster in your classroom for reference and revision.
- Engage learners with real-world context and an unusual form of diamond with a downloadable news story and summary slide.
More resources
- Use allotropes of carbon structure strips (rsc.li/4eO3KhY) to help students develop their literacy skills in science.
- To identify learning gaps and misconceptions, use differentiated worksheets (rsc.li/3zRlZnP) for students.
- Enrich your students’ learning by sharing the story of Harry Kroto (rsc.li/3zTSIsM), a co-discoverer of buckminsterfullerene, C60.
Harry Lord
This helps students visually see that each carbon will have one electron left and reinforces the idea of delocalisation as these electrons can carry the charge through the structure












No comments yet