Help students find a way through the complexity of atomic modelling
Throughout the history of scientific discovery, chemists and physicists have proposed many different models for the structure of the atom. We use several of these models at different levels of chemistry education. Some models stick in students’ minds more than others.
Developing the atomic model
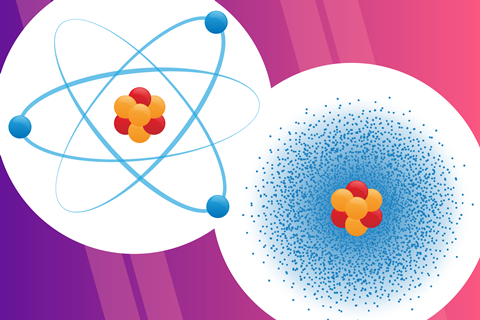
In the Bohr model, electrons occupy orbits at specific distances from the nucleus. It is easy to express and visually represent.
Throughout scientific discovery, chemists and physicists have proposed many different models for the structure of the atom. We use several of these models at different levels of chemistry education. Some models stick in students’ minds more than others.
Developing the atomic model
In the Bohr model, electrons occupy orbits at specific distances from the nucleus. It is easy to express and visually represent. Use our infographic poster and the accompanying worksheet to help your learners draw these diagrams (rsc.li/3WESr7e).
With the introduction of quantum theory, the atomic model becomes more complex. We express the probability of finding electrons in a specific region with the concept of an electron cloud. We describe the most probable location of an electron around an atom as an orbital. This is less easy to express and visually represent than the Bohr model. So, concepts related to the electron cloud, such as uncertainty about the position of electrons, quantised energy levels, electron density and probability, are more challenging for students to understand.
Teaching tips
- Make the abstract nature of atomic theory more concrete through appropriate analogies. For example, relate the probability of finding an electron in an orbital to rolling a certain number on a die, or visualise electron clouds using interactive simulations.
- Ask students to compare the Bohr model to the currently accepted atomic model, highlighting inadequacies in the former.
- Ask questions about each related concept of the electron cloud to be sure your students understand.
- Help students make connections by presenting any explanation of conceptual information with an appropriate visual representation, such as a diagram or graph.
- Use the questions from this study as part of your classroom practice.
A recent study investigated the mental models that post-16 students hold of the electron cloud. It used a case study approach, involving a single school in Turkey and 72 students. All students had previously learned about atomic theory in their chemistry classes.
With the introduction of quantum theory, the atomic model becomes more complex. We express the probability of finding electrons in a specific region with the concept of an electron cloud. We describe the most probable location of an electron around an atom as an orbital. This is less easy to express and visually represent than the Bohr model. So, concepts related to the electron cloud, such as uncertainty about the position of electrons, quantised energy levels, electron density and probability, are more challenging for students to understand.
A recent study investigated the mental models that post-16 students hold of the electron cloud. It used a case study approach, involving a single school in Turkey and 72 students. All students had previously learned about atomic theory in their chemistry classes.
The study collected data using seven open-ended questions, which covered three aspects of the electron cloud: conceptual, relational and visual.
Only 6% provided answers aligned with the full, rather than partial, scientific model
It measured students’ conceptual understanding of electron clouds by assessing their understanding of key ideas, such as the meaning of the term orbital. It measured relational understanding through comparisons between the outdated Bohr model and more contemporary atomic theory. It assessed learners’ visual understanding by asking them to draw representations, such as graphs or diagrams, of key concepts.
Three mental models
Analysis of student responses to these questions resulted in the study proposing three mental models:
- The scientifically accepted electron cloud model, which is either fully or partially adopted by students.
- The hybrid/synthesis electron cloud model, which is deficient in at least one key aspect (conceptual, relational or visual).
- The primitive model, which is deficient in all key aspects.
The study identified about 22% of students using the electron cloud model, with only about 6% providing answers aligned with the full, rather than partial, scientific model. Most students, about 75%, used the hybrid/synthesised electron cloud model, with the largest percentage of these using a model that was mostly conceptual. Only about 3% of students used the primitive model.
The results of this study show that students have problems in fully understanding the current scientifically accepted model of atomic theory, particularly relating to electron clouds.
Teaching tips
- Make the abstract nature of atomic theory more concrete through appropriate analogies. For example, relate the probability of finding an electron in an orbital to rolling a certain number on a die or visualise electron clouds using interactive simulations: bit.ly/4dtRIdh
- Ask students to compare the Bohr model to the currently accepted atomic model, highlighting inadequacies in the former.
- Ask questions about each related concept of the electron cloud to be sure your students understand.
- Help students make connections by presenting any explanation of conceptual information with an appropriate visual representation, such as a diagram or graph.
- Use the questions from this study as part of your classroom practice.
Fraser Scott
Reference
S Çalış, Chem. Educ. Res. Pract., 2024, doi.org/10.1039/d4rp00083h
References
S Çalış, Chem. Educ. Res. Pract., 2024, 25, 1105 (doi: 10.1039/d4rp00083h)














No comments yet