Understanding post-16 atomic models can inform your teaching of 14–16 students
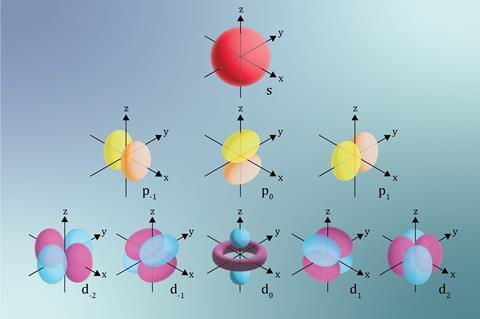
Describing the structure of atoms relies on models. The models used at 11–14, 14–16 and post-16 become increasingly more complex. Understanding the post-16 models is useful when teaching 14–16 students, to minimise student misconceptions.
At 14–16, we model the atom as a tiny, massive, positively-charged nucleus, surrounded by shells of electrons. This model primarily stems from the work of Hans Geiger, Ernest Marsden, Ernest Rutherford and Niels Bohr. Rutherford inferred the existence of atomic nuclei from the gold foil experiments carried out by Geiger and Marsden. Bohr, in his interpretation of atomic spectra, proposed that electrons exist in ‘orbits’ (now shells) with fixed energy levels, and that electrons can move between the orbits when energy is absorbed or emitted.
Potential issues with the model we use come from students wondering ‘what are the shells made from?’, and the inability of the model to account for the three-dimensional shape of simple molecules.
What the model lacks
Ideas that can’t be explained using the 14–16 model of electronic structure include:
- bonding in H2SO4
- why transition metals are highly coloured
- why zinc isn’t really a transition element
- why oxygen is paramagnetic
- why He2+ can exist, but He2 can’t.
What you need to know
Why do we stop at calcium when describing electronic structures at 14–16? This is a direct consequence of the simplified model we use. We often say there are a maximum of two electrons in the first shell, and eight in the subsequent shells. At calcium (20 electrons, so 2,8,8,2) we hit the limit of this model, as the electronic structure of scandium (21 electrons) is 2,8,9,2, rather than the expected 2,8,8,3 from the model we use.
The reason for this apparent anomaly becomes clear when we look at the more sophisticated model used for post-16 chemistry. While we usually consider electrons to be particles, they are more accurately described as quantum particles. Electrons have both particle-like and wave-like properties. Using the rules of quantum mechanics, we can derive four numbers which identify the position and energy of electrons in atoms.
These quantum numbers help to describe the electron shells, which have a complex substructure, being made of subshells, which themselves are made of orbitals. Within each orbital, two electrons can exist.
Each shell has one more subshell than the previous shell, and each subshell has two more orbitals then the previous subshell. For example, the first shell is formed of one s-type subshell, made up of one orbital that we call 1s. Two electrons with opposite spin can exist in this orbital. This accounts for the electronic structure of hydrogen (1 or 1s1) and helium (2 or 1s2).
The second shell is formed of an s-type subshell (one orbital, hence two electrons, called 2s) and a p-type subshell (three orbitals, hence six electrons, called 2p). This accounts for the electronic structures of beryllium (2,1 or 1s22s1) through to neon (2,8 or 1s22s22p6).
The third shell is formed of one 3s orbital, three 3p orbitals and five 3d orbitals. There are complex rules explaining the order of the filling of the orbitals. As the electrons fill the 3s and 3p orbitals, we account for sodium through to argon. However, in potassium and calcium atoms, the 3d orbitals are higher in energy than the 4s, so the latter orbitals fill with electrons first. At scandium, the relative energy of the 3d and 4s orbitals are reversed, and one of its 21 electrons goes in a 3d orbital. The particular shapes of the orbitals, shown in the figure above, directly impact the shapes and properties of molecules. For example, s orbitals are generally spherical and p orbitals look a bit like dumb-bells. How these orbitals overlap when electrons are shared in covalent bonds dictates the strength and type of covalent bonds formed.
Teaching my 14–16 students, I won’t go into the full details of subshells and orbitals (unless they are particularly keen). However, I will talk explicitly about the limitations of the model we are using, and why we make the apparently arbitrary decisions about which elements’ electronic structures to care about. Keith Taber’s An analogy for the atom probe is a useful resource if you want to take these discussions further.
The third shell is formed of one 3s orbital, three 3p orbitals and five 3d orbitals. There are complex rules explaining the order of the filling of the orbitals. As the electrons fill the 3s and 3p orbitals, we account for sodium through to argon. However, in K and Ca atoms, the 3d orbitals are higher in energy than the 4s, so the latter orbitals fill with electrons first. At scandium, the relative energy of the 3d and 4s orbitals are reversed, and one of its 21 electrons goes in a 3d orbital. The particular shapes of the orbitals, shown in the figure below, directly impact the shapes and properties of molecules. For example, s orbitals are generally spherical and p orbitals look a bit like dumb-bells. How these orbitals overlap when electrons are shared in covalent bonds dictates the strength and type of covalent bonds formed.
Teaching my 14–16 students, I won’t go into the full details of subshells and orbitals (unless they are particularly keen). However, I will talk explicitly about the limitations of the model we are using, and why we make the apparently arbitrary decisions about which elements’ electronic structures to care about. Keith Taber’s An analogy for the atom (rsc.li/3PIL6fk) probe is a useful resource if you want to take these discussions further.
Clearing up misconceptions
Students at 14–16 will sometimes ponder the structure of the periodic table, and wonder at the apparent arbitrariness of the layout. Why are there only two elements in the first row, then eight in the second and third? Why are these rows divided into two and six like that?
Knowing something about subshells and orbitals starts to draw back the conceptual curtain. If shell one is made of only one orbital, it makes sense for there to be only two elements. If shell two is made of four orbitals, it makes sense for there to be eight elements. And so on. You can even refer to the blocks by their formal names, from left to right as s, d and p (with f usually underneath for convenience).
As always with models, they are a description of reality at a level useful for a purpose. For the majority of students, understanding electron structures up to calcium is sufficient, so there is no direct need to introduce a more complex model. However, there are some concepts at 14–16 that can’t be explained by the model we use, and so it can be useful to allude to the more sophisticated model.
Suggestions for teaching atomic models at 14–16
- Be upfront with your students that 2,8,8,2 is a model, a rule of thumb – useful for explaining some ideas, but only showing some of the surface detail of what is really happening.
- Ask your students how they are visualising atoms. Do they see them as having physical shells that the electrons sit in?
- Try to be careful and consistent with your language. Avoid jumping between ‘energy level’ and ‘electron shell’ when discussing these ideas.
- Use analogies when helping students form their concepts. For example, electrons fill shells like hotel guests fill rooms in a high-rise hotel. Higher energy shells (higher floors) are empty (available) for electrons (guests) to move into when needed.
- Encourage interested students to read further – direct them to general post-16 textbooks (eg Hill and Holman’s Chemistry in Context) or websites like chemguide.co.uk.
- Ensure your students can write concise and coherent responses to exam-style questions such as ‘describe and explain the electronic structure of a calcium atom’.
Suggestions for teaching atomic models at 14–16
- Be upfront with your students that 2,8,8,2 is a model, a rule of thumb – useful for explaining some ideas, but only showing some of the surface detail of what is really happening.
- Ask your students how they are visualising atoms. Do they see them as having physical shells that the electrons sit in?
- Try to be careful and consistent with your language. Avoid jumping between ‘energy level’ and ‘electron shell’ when discussing these ideas.
- Use analogies when helping students form their concepts. For example, electrons fill shells like hotel guests fill rooms in a high-rise hotel. Higher energy shells (higher floors) are empty (available) for electrons (guests) to move into when needed.
- Encourage interested students to read further – direct them to general post-16 textbooks (eg Hill and Holman’s Chemistry in Context) or websites like chemguide.co.uk.
- Ensure your students can write concise and coherent responses to exam-style questions such as ‘describe and explain the electronic structure of a calcium atom’.
Resources for your classroom
See all of our supporting resources for teaching this topic at a glance in our atomic model resources package for learners aged 14–16.
- Encourage students to research the key scientists involved in the development of our understanding of atomic structure. A good starting place is the biography of Niels Bohr on the Nobel prize website.
- Use this Starter for ten activity to quiz your students on the atomic structure of atoms.
- Bust students’ misconceptions about chemical stability with these ready-to-use classroom resources.
- Here’s how to teach atomic structure to 11–14 and post-16 students.
Resources for your classroom
- Encourage students to research the key scientists involved in the development of our understanding of atomic structure. Start with a biography of Niels Bohr: bit.ly/3w4mYMj
- Use this classroom activity to quiz your students on the atomic structure of atoms: rsc.li/3zGCpwx
- Bust students’ misconceptions about chemical stability with these ready-to-use classroom resources: rsc.li/3zBKNNK
David Paterson teaches chemistry at Aldenham School and is a chemistry adviser at CLEAPSS













2 readers' comments