Boost your 14–16 students’ confidence drawing electron configuration diagrams with these easy to follow steps
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. This is sometimes called the Bohr, or the ‘solar system’, model.
-

Infographic poster and fact sheet
Display the poster in your classroom or on a projector. Alternatively print it and use as a handout.
- Poster with the atomic number at the top as pdf A4 single pages or A3 one page
- Poster with the atomic number at the bottom as pdf A4 single pages or A3 one page
- Fact sheet as MS Word or pdf
-

View and download more infographics


Neils Bohr was the first to suggest the idea that electrons orbit the atom in fixed shells, or energy levels, in 1913. Bohr observed that bursts of energy emitted from hydrogen atoms, visible as light, only occurred at specific wavelengths. He suggested this was due to electrons moving between energy levels rather than being scattered randomly around the nucleus. He was awarded a Nobel prize for his work.
Bohr impressed fellow scientist Rutherford, who discovered the nucleus of the atom, but didn’t win over JJ Thomson whose ‘plum pudding’ model of the atom was replaced by Rutherford’s, then Bohr’s, model
In the Bohr model, there are a few rules that will help you draw accurate diagrams.
- Electrons must occupy the lowest available shell, closest to the nucleus.
- The maximum number of electrons that can fill each shell is:
- two in the first shell,
- eight in the second shell,
- eight in the third shell.
- Calcium, the 20th element, has two further electrons that go in the fourth shell.
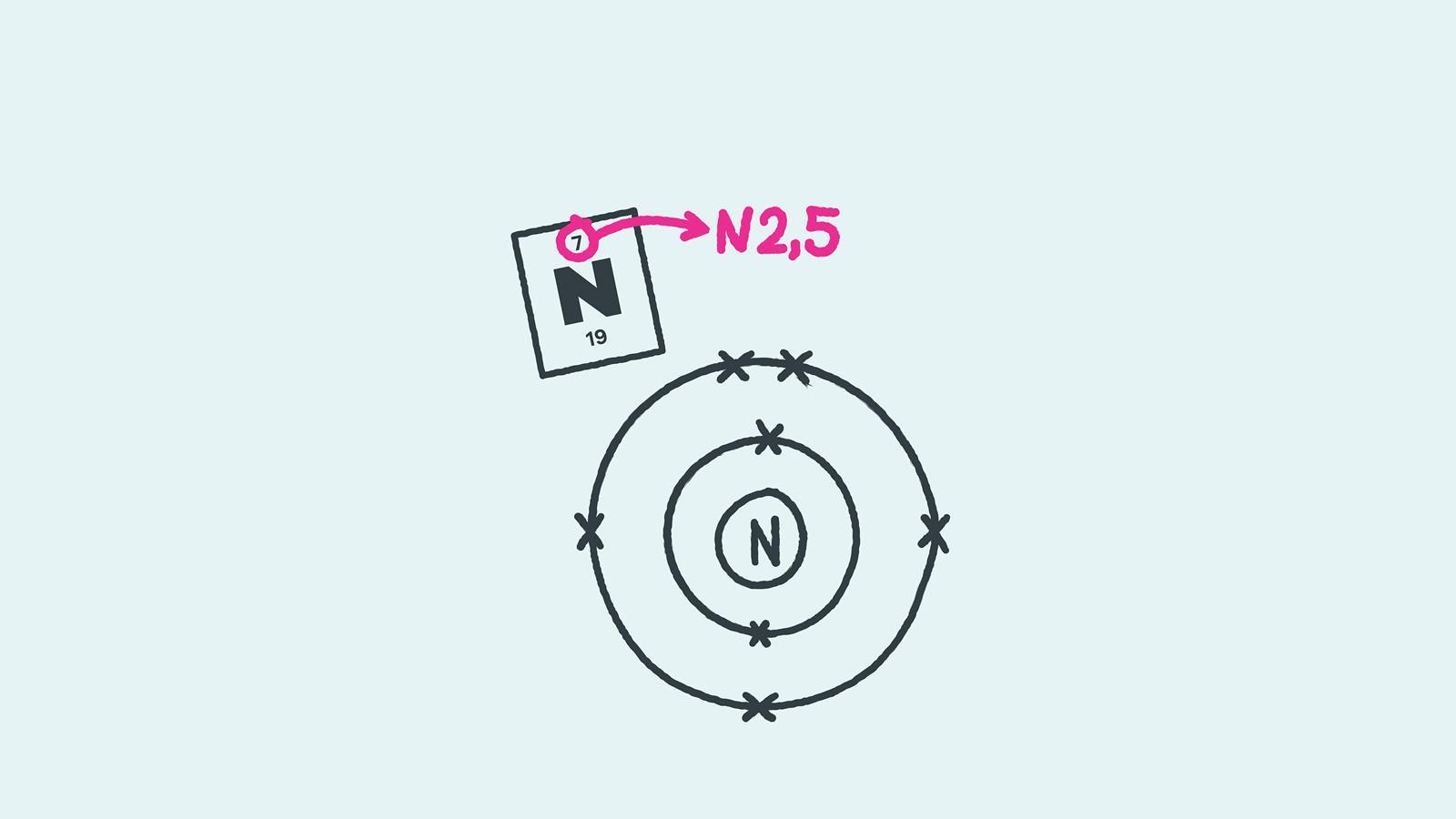
In the shorthand notation for electron configuration, the number of electrons in each shell can be written rather than drawn. Each shell is separated by a full stop or a comma. For nitrogen this would be 2.5 or 2,5 and for calcium this would be 2.8.8.2 or 2,8,8,2.
Did you know …?
The arrangement of an element’s electrons tells you where it is on the periodic table. The number of shells shows which period, or row, it’s in and the number of electrons in the outer shell shows which group it’s in.
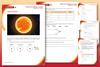
How to draw an electron configuration diagram
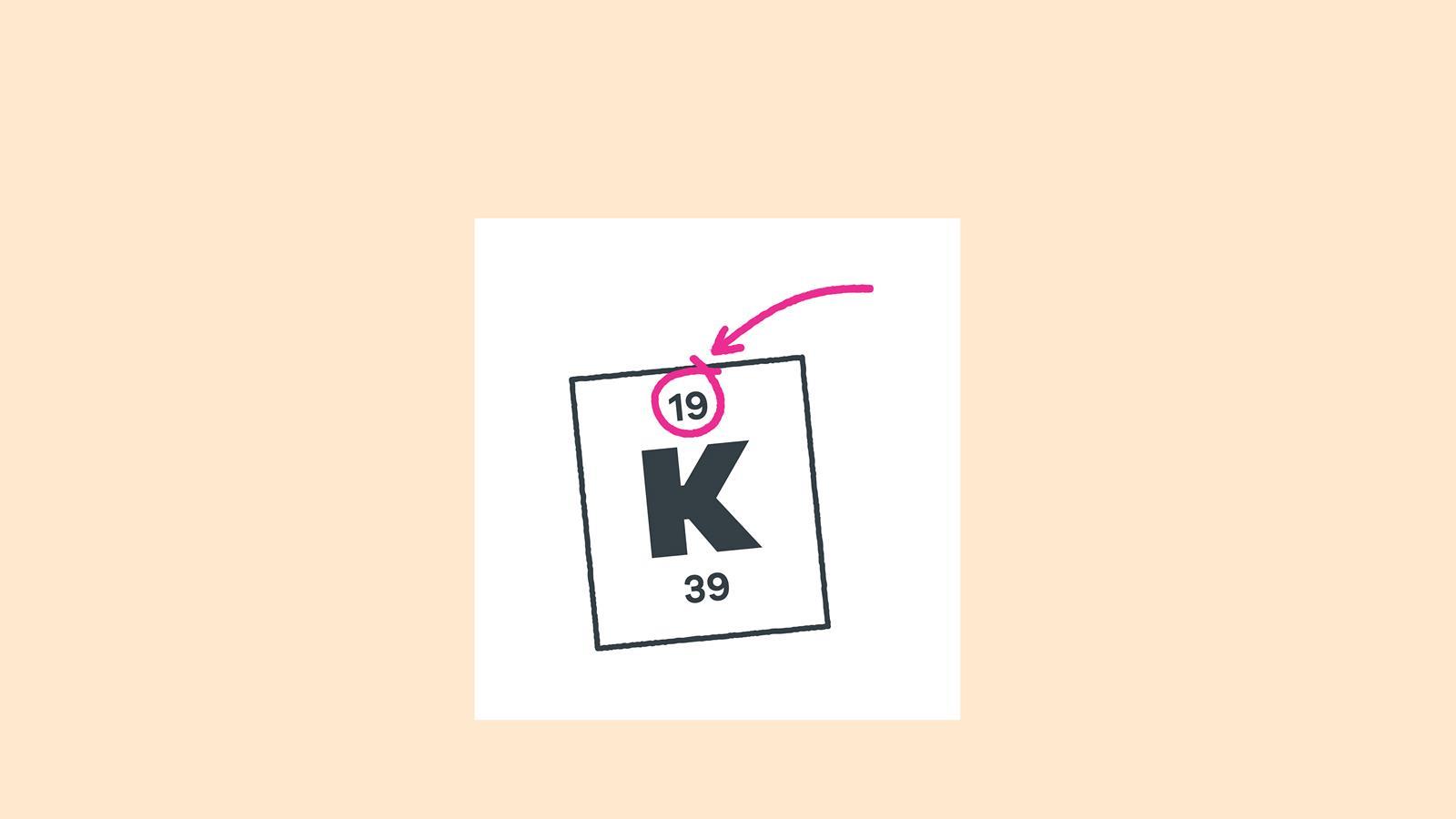
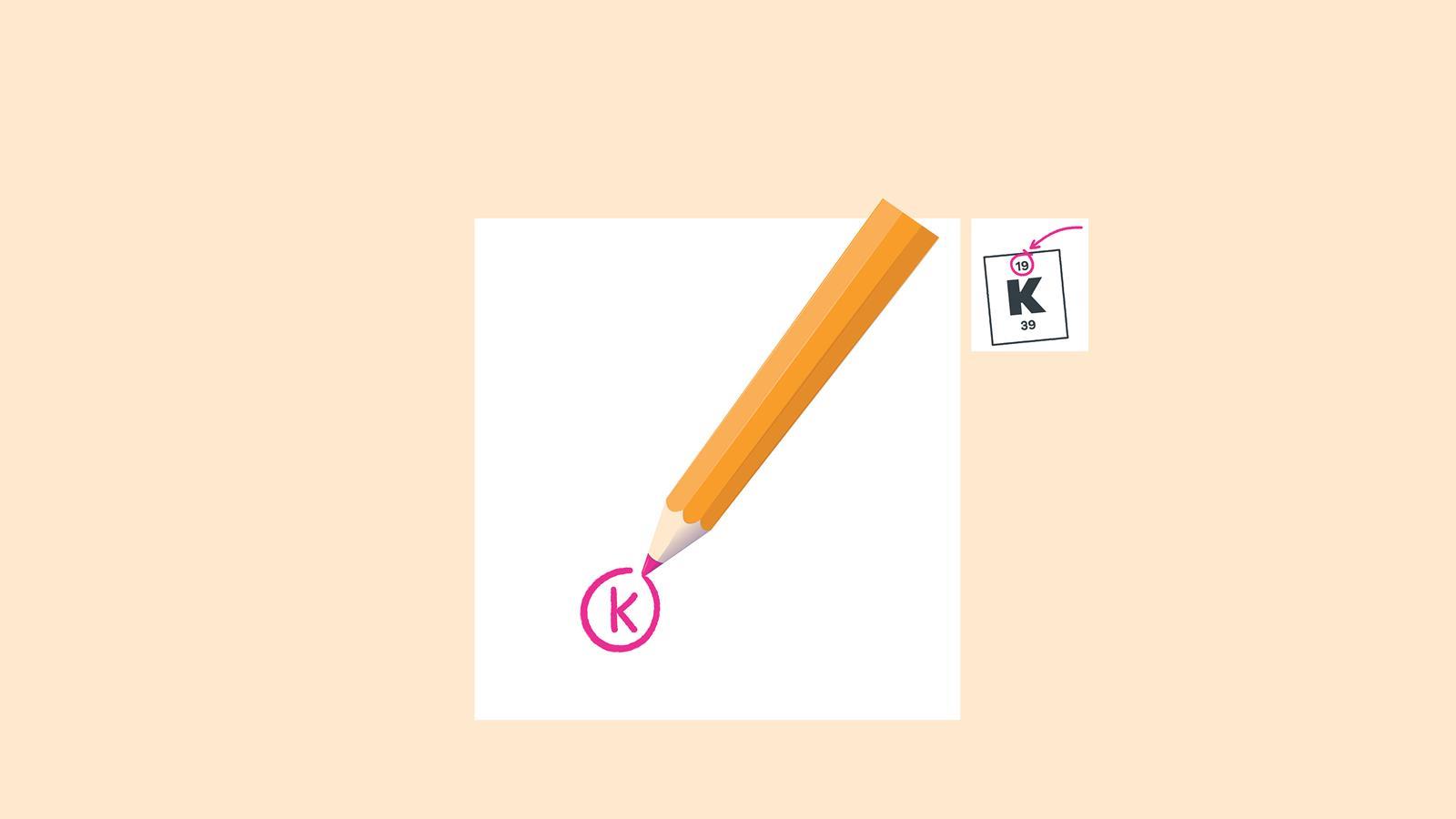
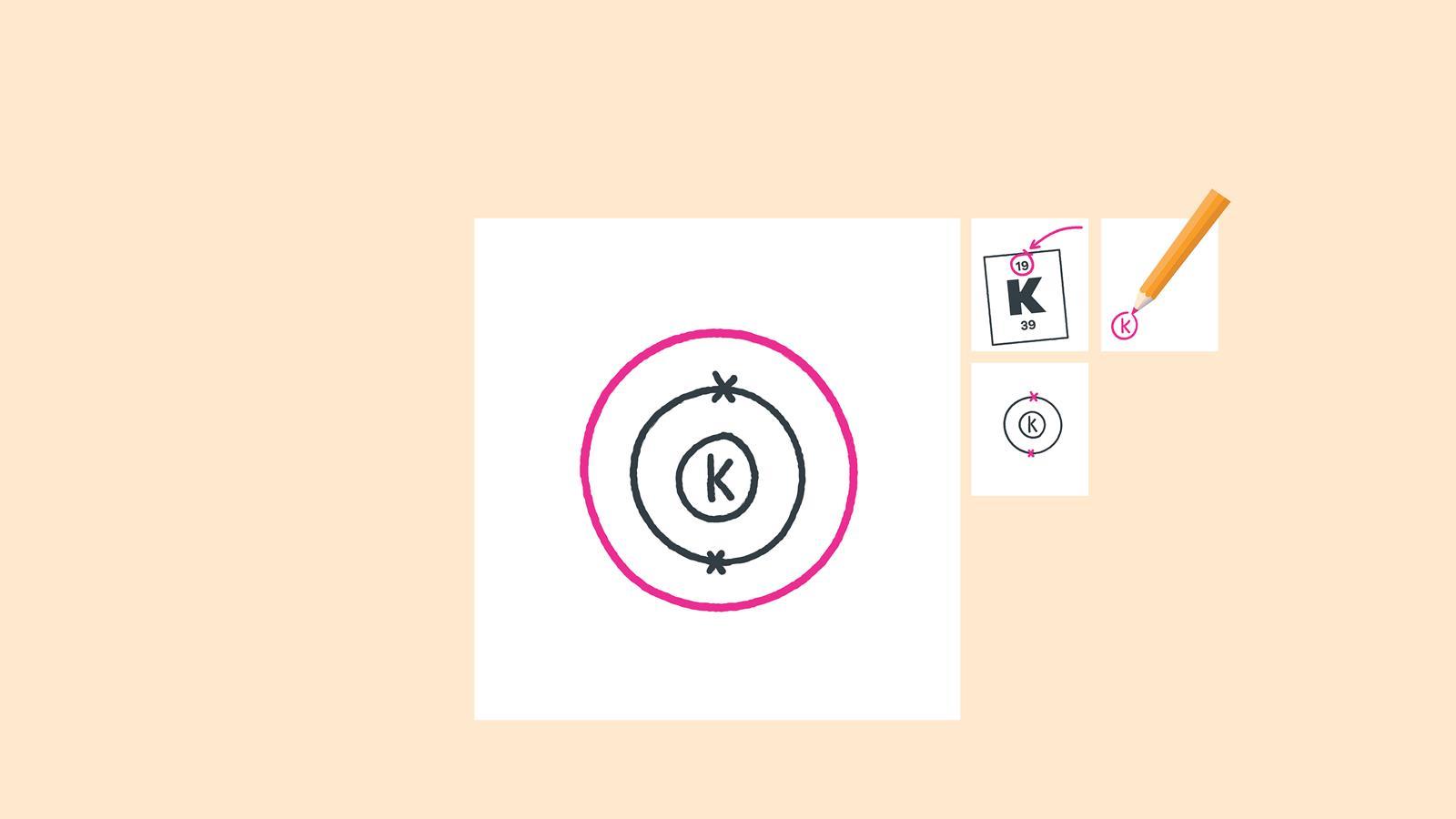
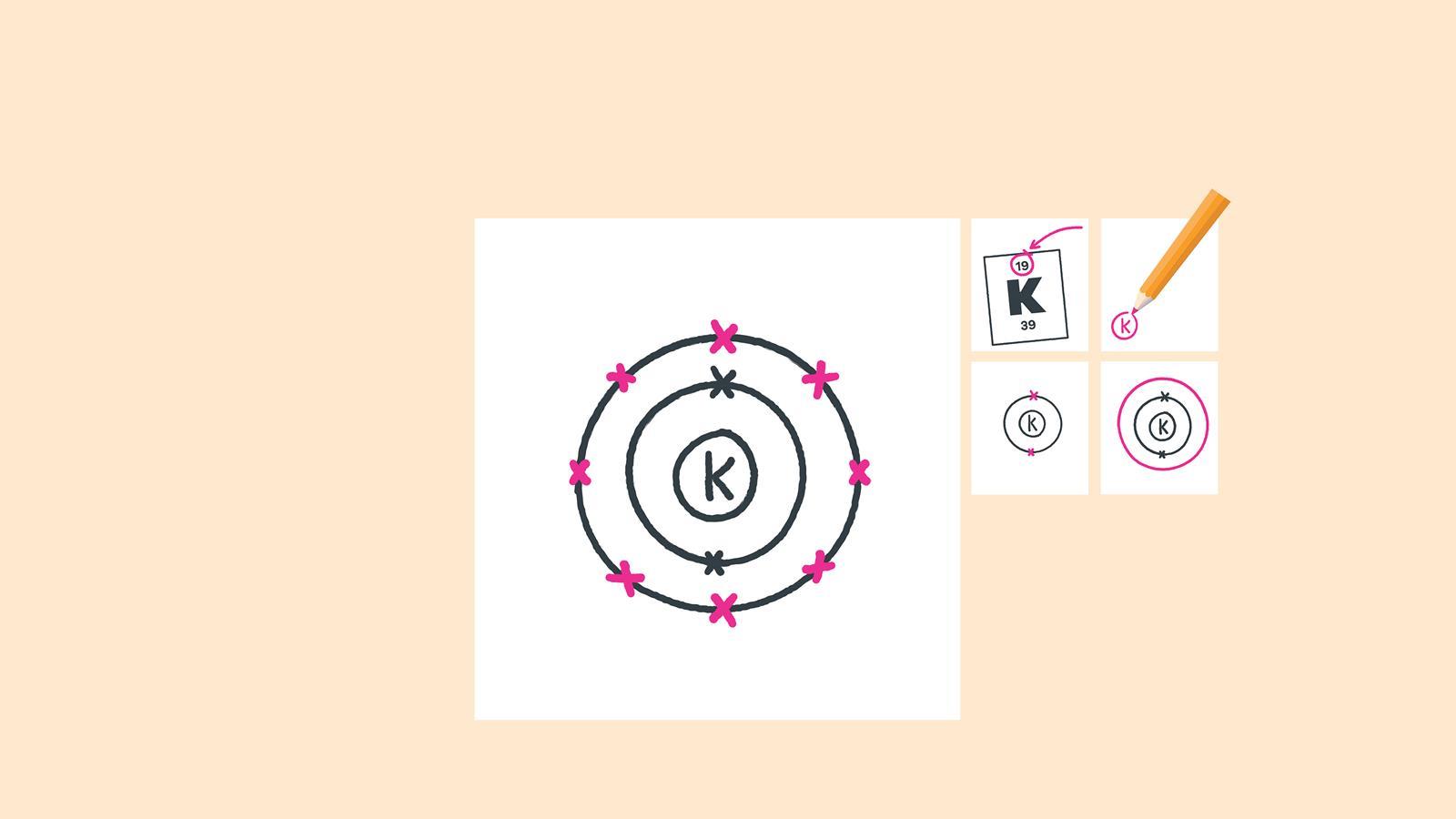
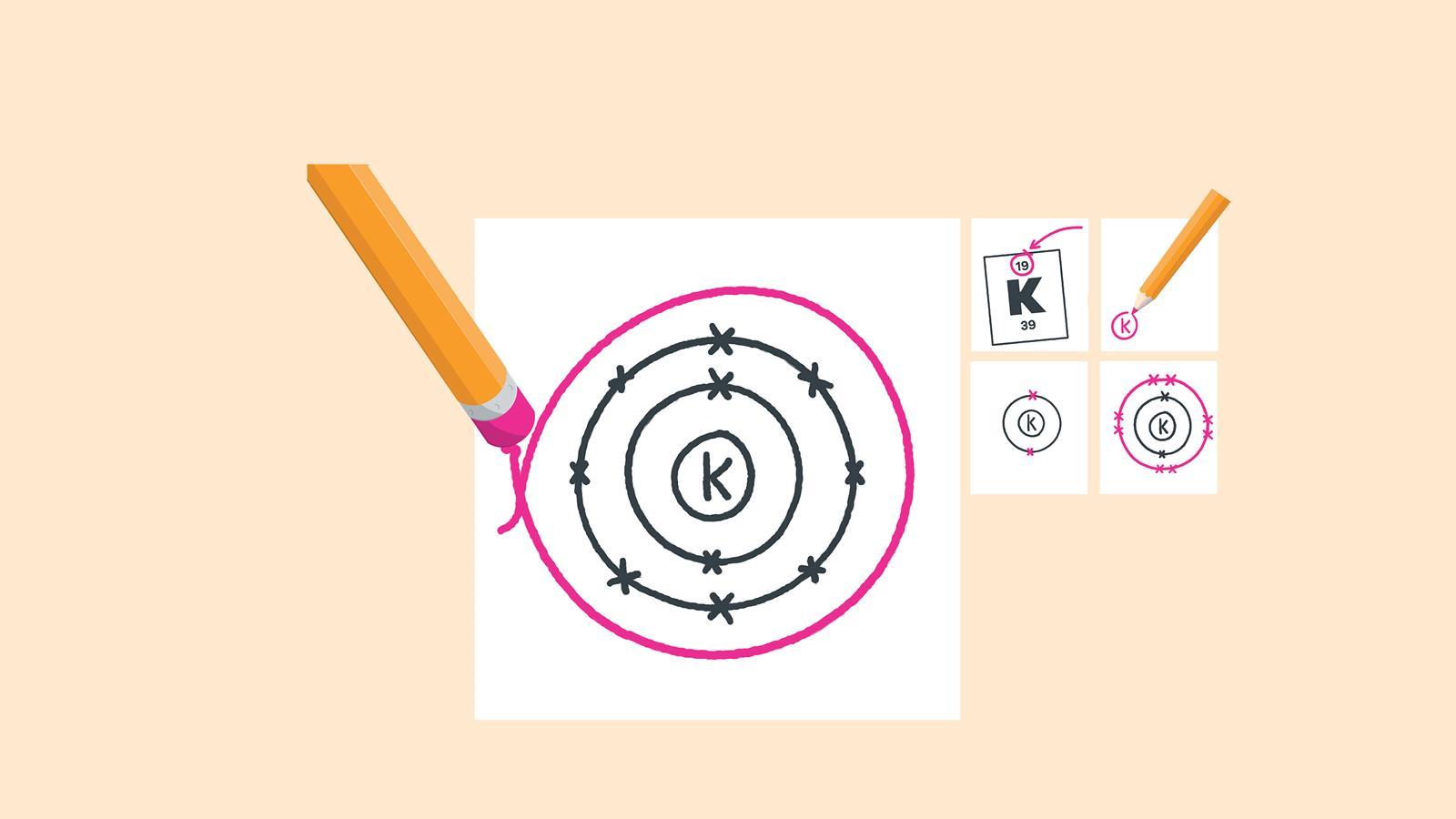
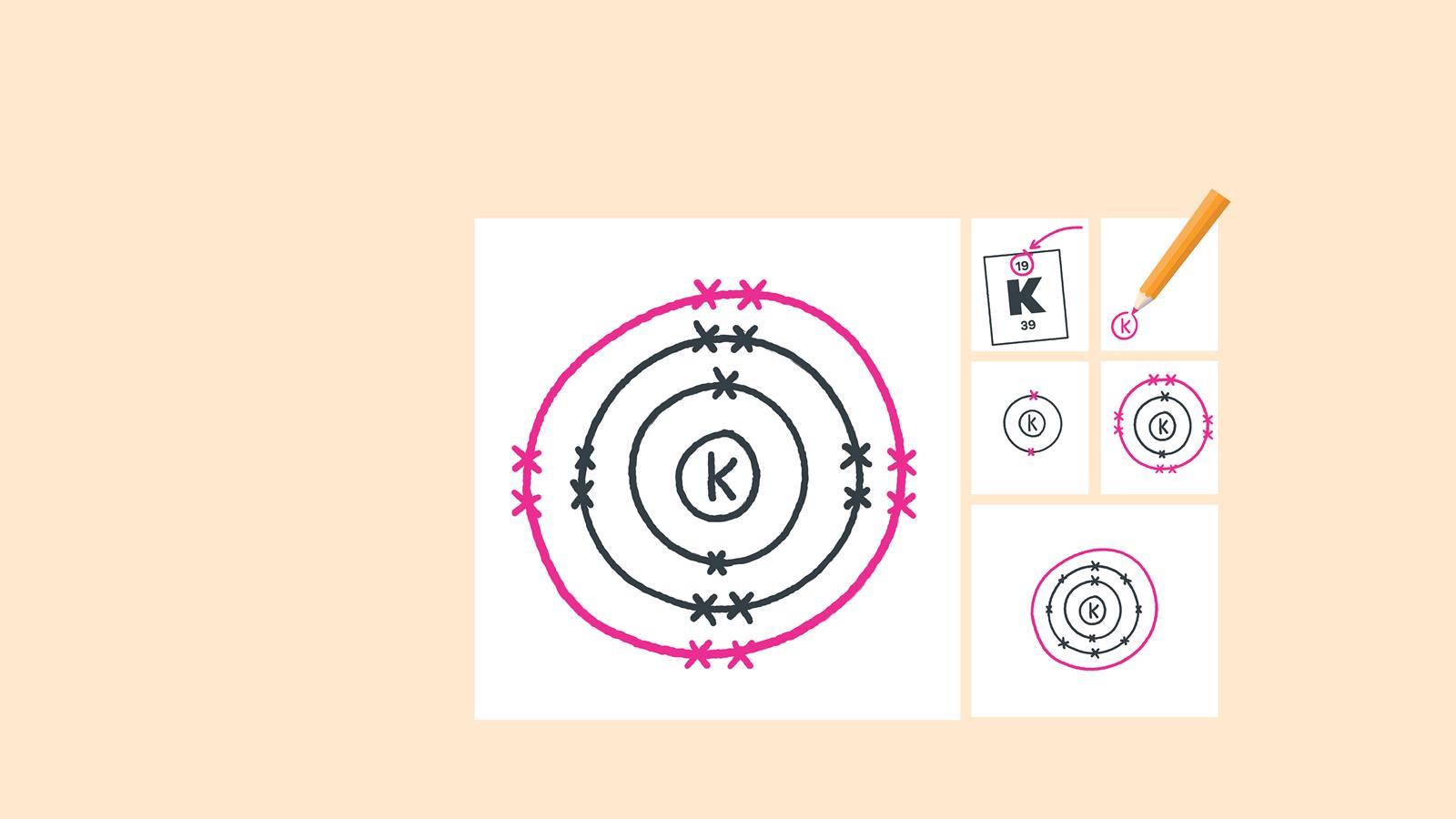
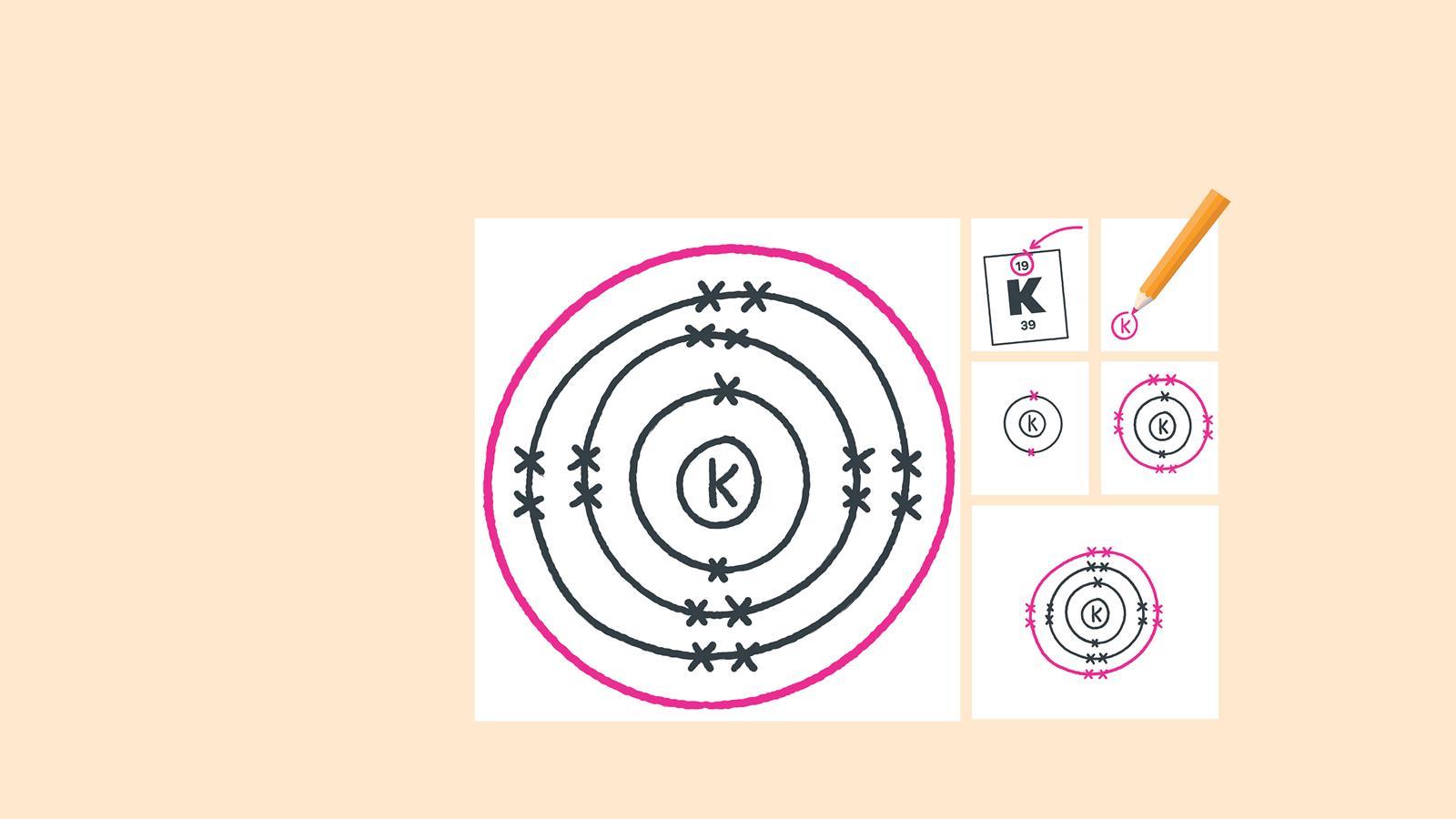
Find the element on the periodic table. The atomic number tells you how many electrons to draw in total. For example, potassium has 19 electrons
Draw a small circle and write the symbol in the centre. This represents the nucleus
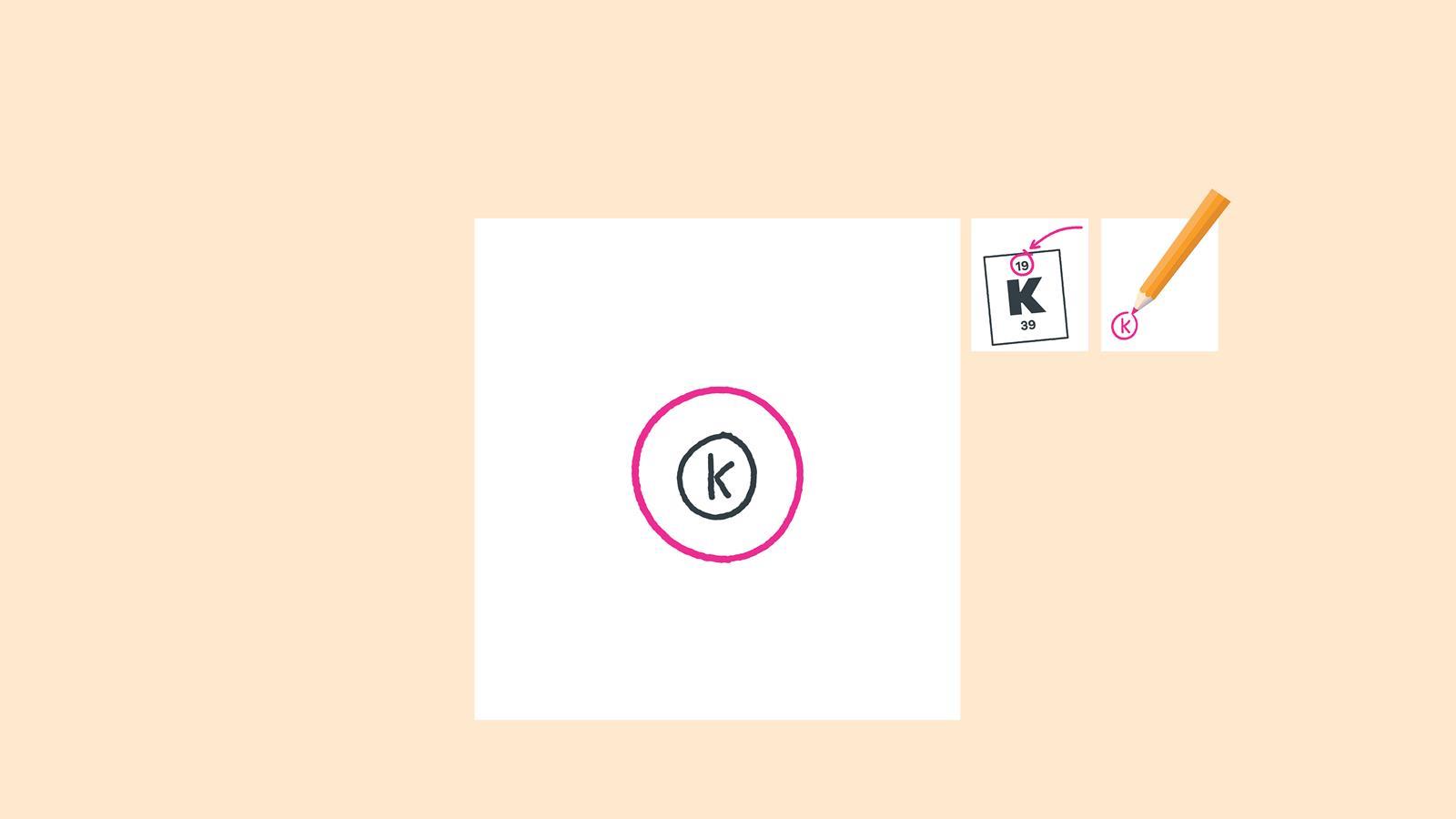
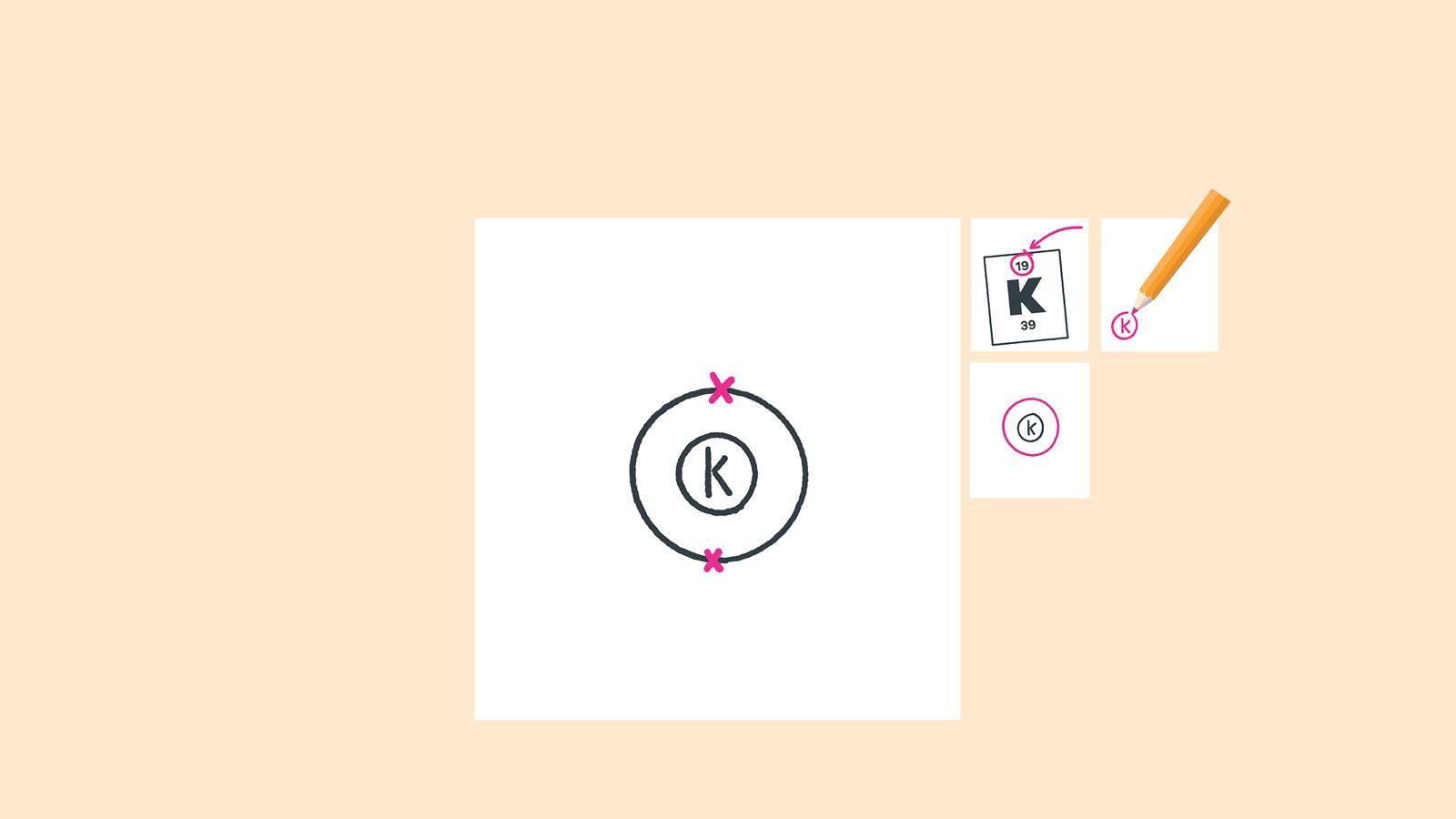
Draw a circle around the nucleus. This is the first electron shell
Add up to two electrons to the first electron shell. Electrons are usually represented by a dot or cross
Draw another circle around the first shell. This is the second shell
Add up to eight electrons to the second shell
Draw another circle around the second electron shell. This is the third shell
Add up to eight electrons to the third shell
Draw the last circle around the third shell. This is the fourth electron shell
Add up to two electrons to the fourth electron shell. For potassium, only one electron is added to this shell
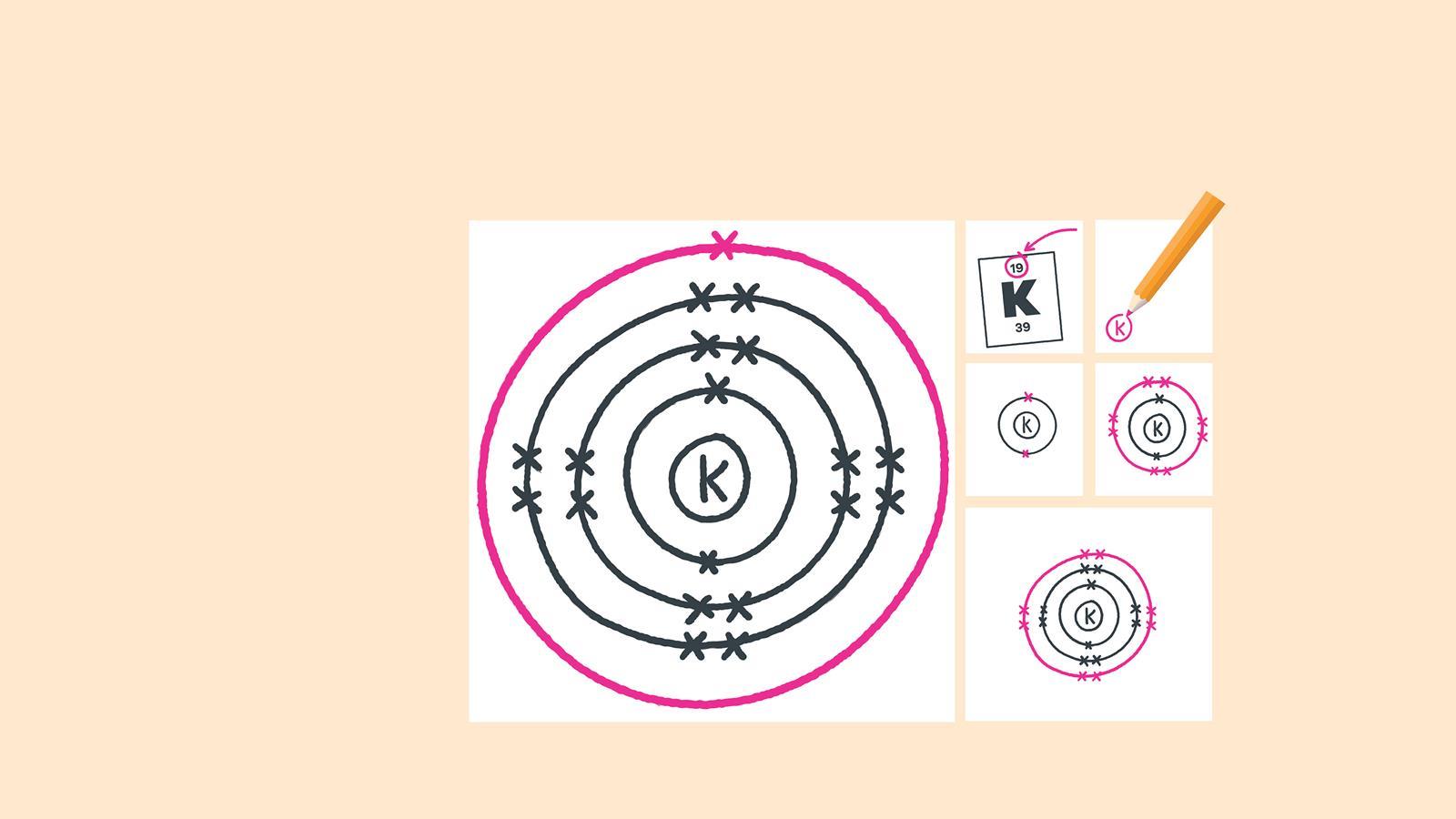
Now draw the first 20 elements
Use the steps above to draw electron configuration diagrams of the first 20 elements
The position of electrons may depend on what you need to draw next
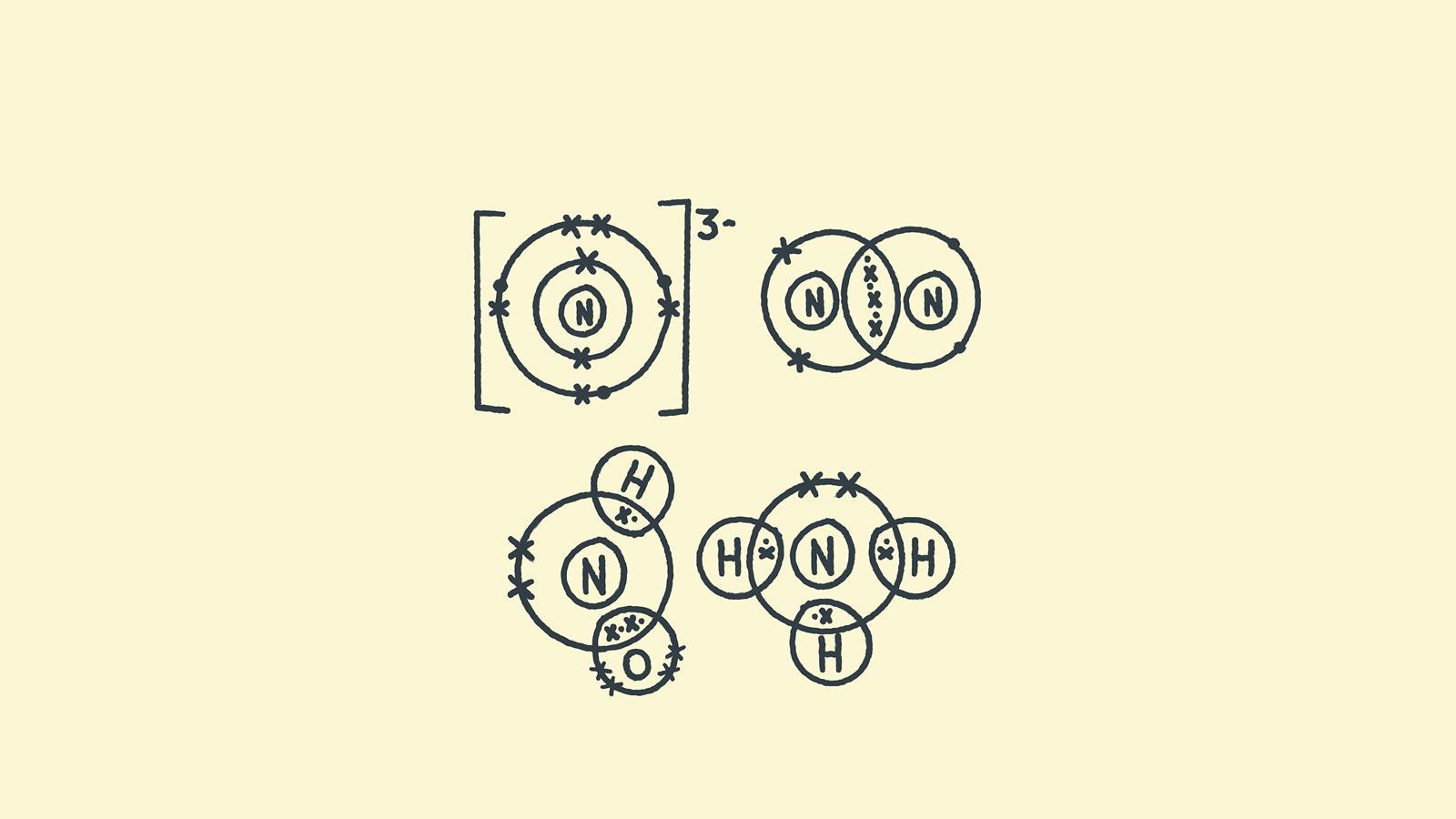
Want other step-by-step guides to drawing bonds? Check out these articles with resources on drawing dot and cross diagrams for:
All illustrations © Dan Bright
Downloads
Electron configuration diagrams poster (atomic number top) A4 single pages
PDF, Size 5.34 mbElectron configuration diagrams poster (atomic number top) A3
PDF, Size 5.34 mbElectron configuration diagrams poster (atomic number bottom) A4 single pages
PDF, Size 5.38 mbElectron configuration diagrams poster (atomic number bottom) A3
PDF, Size 5.75 mbElectron configuration diagrams fact sheet
Editable handout | Word, Size 72.42 kbElectron configuration diagrams fact sheet
Handout | PDF, Size 0.14 mbConfiguration confusion - student sheet
Editable handout | Word, Size 0.37 mbConfiguration confusion - student sheet
Handout | PDF, Size 0.35 mbConfiguration confusion - answer sheet
Editable handout | Word, Size 0.75 mbConfiguration confusion - answer sheet
Handout | PDF, Size 0.66 mb












































5 readers' comments