Use this poster, fact sheet and peer-assessment activity to help your post-16 students understand orbitals and shells
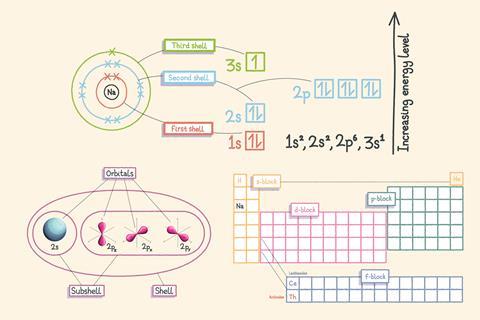
We describe most chemical changes in terms of a rearrangement of electrons. It’s therefore crucial to have an accurate understanding of the arrangement of electrons (the electron configuration) in atoms and ions. Electron configurations give us insight into the bonds that atoms are likely to form and the relative stability of ions.
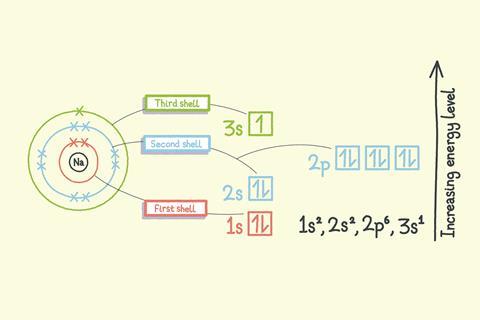
Shells are the allowed energy levels of electrons. The first shell has the lowest energy and the energies increase as the electrons get further away from the positively charged nucleus.
Download this
Infographic poster and fact sheet, student worksheet and teacher notes for peer-assessment activity. Display the poster in your classroom or on a projector. Alternatively print it and use as a handout.
Use the accompanying activity to check your post-16 learners’ understanding by asking them to correct and improve incorrect electron configurations.
Subshells
All shells are subdivided into different energy levels called subshells, with the exception of the first shell which is not subdivided. The second shell is divided into two subshells: s and p. The third shell is divided into three subshells: s, p and d.
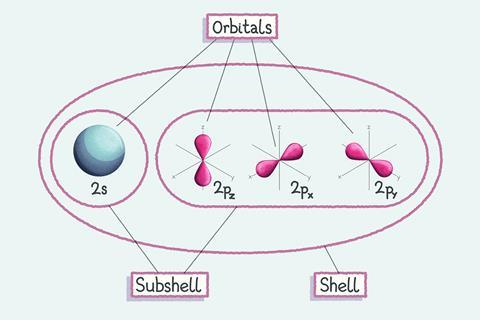
Orbitals
Each subshell contains different shaped orbitals – regions in space where we are likely to find electrons. Each orbital can contain just two electrons, which must have opposite spin from each other.
More resources
- Display the post-16 poster in your classroom next to the 14–16 infographic How to draw electron configuration diagrams to illustrate how ideas and models change with an increase in complexity.
- Use this CPD article to boost your understanding of post-16 atomic models, and minimise student misconceptions at 14–16.
- Use this teacher-designed card game to teach electronic configuration and ionic compound formation.
- Want to know how this fits into the rest of the topic? Here’s how to teach atomic structure and periodicity at post-16.
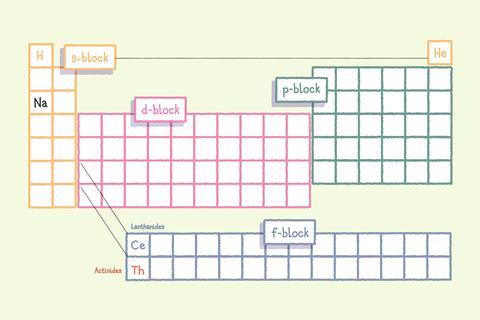
Sodium (Na) is in the s-block of the periodic table, which is two elements wide. The p-block contains elements with their highest energy electrons in the p subshell and is six elements wide.
Did you know …?
The labels s, p, d and f come from the appearance of lines in line spectra. They stand for sharp, principal, diffuse and fundamental.
All illustrations © Dan Bright
Want more posters with activities for your post-16 learners?
Downloads
Electron configuration infographic poster
PDF, Size 0.43 mbElectron configuration fact sheet
Handout | PDF, Size 0.11 mbElectron configuration fact sheet
Editable handout | Word, Size 0.45 mbCorrecting configurations student worksheet
Handout | PDF, Size 0.16 mbCorrecting configurations student worksheet
Editable handout | Word, Size 0.45 mbCorrecting configurations teacher notes
Handout | PDF, Size 0.21 mbCorrecting configurations teacher notes
Editable handout | Word, Size 0.45 mb














1 Reader's comment