Does students’ goal-oriented approach get in the way of better problem-solving strategies?
When it comes to ‘learning’ organic chemistry, students often resort to rote memorisation instead of developing an understanding of the underlying chemistry. This isn’t entirely unexpected as predicting where those curly arrows go to transform reactants into products is hard!
Unfortunately, it’s precisely this way of thinking that contributes to the challenge. When students are asked why they are proposing a particular mechanistic step, they often reply: ‘it gets me to the product.’ This means-end-analysis does allow problem solvers to reduce the gap between initial and goal states (ie reactants and products). However, could this preoccupation with ‘getting to the product’ get in the way of other, better problem-solving strategies?
A means to an end
Research from the US has provided interesting insights into students’ approaches to solving mechanistic organic chemistry questions. The study involved 24 undergraduate students who were interviewed as they completed a mechanistic reasoning task.
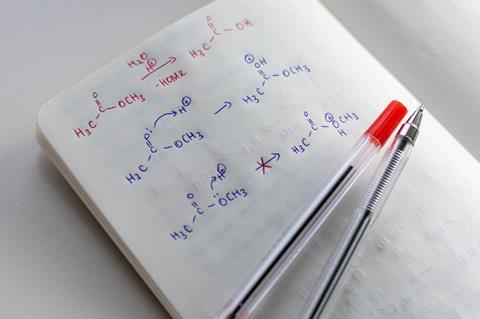
Participants were asked to propose the curly arrow mechanism of an elementary reaction step for several examples of reactions. Sometimes they were provided with the product of a reaction, and sometimes they had to reason without it.
When participants were not given the product of the reaction, their reasoning processes included at least some understanding of the chemical properties of the reactants, such as, identifying the most nucleophilic site. However, when they were later provided with the product of the same reaction, the students engaged in means-end-analysis to attempt to get to the product, and changed their first, correct, answers to incorrect ones because they paid less attention to the chemical properties of the reactants.
One particular example from the study clearly illustrates this shift in the students’ reasoning. Students were shown methyl methanoate and a hydrogen ion as reactants, the set-up for an ester hydrolysis, and asked to draw the curly arrow mechanism for the next step. When they had not been given the product, 23 of 24 participants correctly proposed that the carbonyl oxygen would be protonated in the next step. However, when the product structures were provided (methanoic acid and methanol), 22 of 24 participants proposed that the protonation would occur on the other oxygen, as they were concerned with making this a good leaving group and forming the methanol product.
This study suggests that the mode of presentation of curly arrow tasks may interfere with students’ ability to reason mechanistically.
Teaching tips
A knowledge of the reaction product can help in proposing a sound curly arrow mechanism, but it should not be the only strategy that students employ. As educators, we should be particularly aware of this during the initial instruction phase of mechanistic reasoning.
- Explicitly modelling our thought processes may prevent students adopting only means-end-analysis.
- We should encourage students to write down intermediate solutions to mechanistic problems. This will reduce the cognitive-load while they work out the solution.
- We could reinforce this behaviour by specifically awarding marks for intermediate solutions in formative assessments.
- Rather than assessments focusing only on the correct solution, marks could be awarded for an appropriate analysis of the reactivity of the starting materials, such as identifying all the nucleophilic or electrophilic sites and evaluating the likelihood of different reaction pathways.
References
V DeCocq and G Bhattacharyya, Chem. Ed. Res. Pract., 2018. DOI: 10.1039/c8rp00214b









1 Reader's comment