Demonstrations to capture the student's imagination, by Declan Fleming of Pate's Grammar School, Cheltenham.
In this issue: Magical metal
Fusible alloys
When teaching metals there are a number of ways to show how mixing metals together can alter their properties. Fusible alloys (ones which melt at a low temperature) are an attractive and underused opportunity to show how far we can manipulate a material's properties.
Fusible alloys are used for solders, as temperature triggers in sprinkler systems (where the plug is held in place with a fusible alloy which melts in a fire), as mercury replacements (eg galinstan thermometers) and even as experimental coolants in fast neutron reactors.
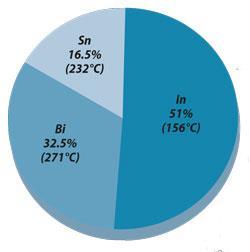
Field's metal is one such alloy which melts at a low temperature (61°C) and, unusually for a fusible alloy, contains neither lead nor cadmium.

Kit
- Sample of Field's metal (~30 g is a good size)
- Ceramic crucible
- 250 cm3 conical flask
- Optional moulding paraphernalia (eg ice cube trays, plasticine, jewellery moulding compound)
Procedure
You can make Field's metal yourself, although it's difficult to obtain small quantities of indium, which can make this prohibitively expensive. Mindsets will supply Field's metal in sensible quantities at approx £1 per gram and it is available through other online sellers.
If you've done this demonstration before, you will potentially have a piece of metal in the shape of the bottom of the container you last used - this will make it difficult for your audience to see what's happening. I normally prepare a specific shape in advance by steaming it in a ceramic crucible over a conical flask. This avoids over-heating the alloy and also avoids having to decant off water if you melt it by submersion.
I prepared a small mould in plasticine by pressing into a piece of acrylic cut on the school's laser cutter. Jewellery moulding compound is available from craft shops. This will produce a reusable mould and you won't need to spend time scratching out bits of plasticine from crevices in the metal. It is possible to produce a mould for a 'magic spoon' but this requires more metal.
In front of the audience
Simply place your piece of metal into a beaker of freshly boiled water and watch it melt!
Teaching goal
Fundamentally the idea here is to show how mixing different elements in an alloy can fine tune its properties to a specific application. You could, however, provide your students with the composition of the mixture and ask them to predict what they expect to see from the alloy: Do students expect the alloy to take on a melting point lower than all three components or higher, or the same as the largest component, or a weighted average of all three? The idea that mixtures can have a lower melting point than a pure substance is covered with younger students and is used with older students when discussing melting point determination.
Storing energy
The result is unexpected both in terms of how depressed the melting point is and the fact that Field's metal is a eutectic alloy - it melts at a specific temperature rather than over a range as will happen with other alloys. This suggests it has a single chemical composition as opposed to being a mixture. This defined, depressed melting point has many novel uses.
New solar molten salt technologies are being developed so that the sun's energy can be used to melt a salt
(eg a eutectic mixture of NaNO3 and KNO3), effectively storing energy for use later (eg at night).
EMLA (eutectic mixture of local anesthetic) is a mixture of lidocaine and prilocaine. Individually, these compounds would be solids at room temperature, but together they melt to an oil at 18°C, avoiding the use of non-aqueous solvents for application and also increasing the dose that can be applied.
Safety
Goggles should be worn for this demonstration. Wash your hands after touching the metal.


















No comments yet