Get started
Watch a demonstration of this experiment and download the technician notes from the Education in Chemistry website: rsc.li/3DSav58
While school electrochemistry focuses on potential difference and its relationship to thermodynamics, when you build a cell, you want it to do something. The classic cell setup with metal strips and beakers of salt solutions connected by a salt bridge creates a voltage you can measure, but the high internal resistance means a single cell won’t power much more than a potato clock. Instead demonstrate this gravity cell configuration in your lessons on electrochemical cells and redox reactions to engage post-16 learners. It keeps everything in one beaker and will power a small motor or buzzer, giving a playful audiovisual indication of how the cell works.
Kit
- Copper(II) sulfate pentahydrate crystals, approx 2 g (harmful if swallowed, corrosive to eyes, skin irritant, very toxic to aquatic life)
- 0.1 M zinc sulfate solution, approx 200 cm3 (eye irritant)
- 1.4 M sulfuric acid, a few drops, optional (skin and eye irritant)
- 250 cm3 beaker
- Zinc sheet, approx 4 × 6 cm*
- Copper sheet, approx 4 × 14 cm*
- Wires
- Crocodile clips
- Voltmeter
- Piezoelectric buzzer
- Small electric motor
*or modelling/jewellery wire (see below)
Health, safety and disposal
- Wear eye protection.
- Use a syringe to extract the upper zinc sulfate solution.
- Add any mixed liquid near the interface to a zinc–copper waste bottle. CLEAPSS members can refer to TL003 for a recommended method for recycling zinc–copper mixtures.
- Leave the saturated copper sulfate solution to crystallise and reuse the crystals.
Health, safety and disposal
- Wear eye protection.
- Use a syringe to extract the upper zinc sulfate solution.
- Add any mixed liquid near the interface to a zinc–copper waste bottle. CLEAPSS members can refer to TL003 for a recommended method for recycling zinc–copper mixtures (bit.ly/3CB4a7g)
- Leave the saturated copper sulfate solution to crystallise and reuse the crystals.
Preparation
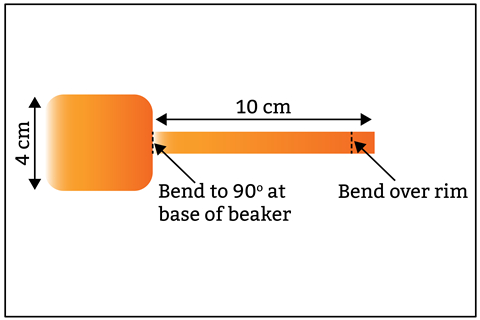
The trick to getting this experiment to work is minimising internal resistance – you need larger pieces of metal than your typical classroom strips. The higher the surface area the better. Reuse or recycle them into smaller pieces for classroom experiments.
Trim a piece of copper large enough to reach the bottom of the 250 cm3 beaker into a paddle shape such that the 4 × 4 cm square base sits on the bottom of the beaker and the narrower 1 × 10 cm handle bends at 90° to the base, leads out of the liquid and bends over the rim of the beaker for connecting to a wire.
Cut a similar paddle from the zinc sheet but the handle need only be a few centimetres long. This allows you to connect a crocodile clip and keep it dry while dunking the zinc just below the surface of the solution.
Alternatively, use uncoated modelling/jewellery wire, widely available from hobby shops. It requires less cutting and is useful for other experiments, like the Lighting up copper demonstration. I moulded a 2 mm diameter copper wire by coiling it around a 100 cm3 beaker and it held its shape at the bottom of the 250 cm3 beaker. You can also buy uncoated zinc modelling wire.
Alternatively, use uncoated modelling/jewellery wire, widely available from hobby shops. It requires less cutting and is useful for other experiments, like the ’Lighting up copper’ demonstration (rsc.li/3D8DUp6). I moulded a 2 mm diameter copper wire by coiling it around a 100 cm3 beaker and it held its shape at the bottom of the 250 cm3 beaker. You can also buy uncoated zinc modelling wire.
In front of the class
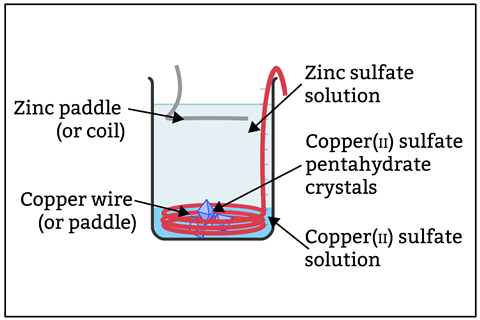
Wear eye protection. Place the copper wire or paddle in the beaker and bend the handle over the rim to secure it. Add 200 cm3 of approx 0.1 M zinc sulfate solution and a few drops of 1.4 M sulfuric acid (optional, see tips). Connect the copper and zinc electrodes to a piezoelectric buzzer. Dip the zinc paddle into the solution without exposing the crocodile clip to the electrolyte. The buzzer will chirp louder as you expose more zinc. The motor is, however, unlikely to run. Drop in a few large crystals (see tips) of copper(II) sulfate pentahydrate. A distinct blue layer will form at the base of the beaker. The voltmeter will read slightly over 1 V. As the crystals dissolve, the buzzer will get louder and eventually, with a little assistance, the motor stutters to life. Stop here or leave the cell running; the solutions will remain separated by their respective densities and the cell’s polarity as long as it is under load.
Tips
Traditionally, deionised water started the cell and the dissolving anode supplied the ions. Starting with zinc sulfate lowers the internal resistance for immediate use.
Adding sulfuric acid greatly improves the conductivity of the solution, which I cover in Shocking revelations 2. The solution will easily run a buzzer without sulfuric acid, but depending on the surface area of your electrodes, it might struggle to get a motor running. Add the acid to the zinc sulfate solution before the lesson to avoid confusing learners as to its purpose.
Technicians often have a supply of larger copper sulfate crystals left over from crystallisation experiments. These are ideal.
Tips
Traditionally, deionised water started the cell and the dissolving anode supplied the zinc ions. Starting with zinc sulfate lowers the internal resistance for immediate use.
Adding sulfuric acid greatly improves the conductivity of the solution, which I cover in ‘Shocking revelations 2’ (rsc.li/40sfK2V). The solution will easily run a buzzer without sulfuric acid, but depending on the surface area of your electrodes, it might struggle to get a motor running. Add the acid to the zinc sulfate solution before the lesson to avoid confusing learners as to its purpose.
Technicians often have a supply of larger copper sulfate crystals left over from crystallisation experiments. These are ideal.
Did you know?
In the 1860s, a Frenchman named Callaud dramatically reduced the internal resistance of the classic zinc–copper Daniell cell. He removed the earthenware pot separating the electrolytes and used their difference in density and the migration of cations to prevent the solutions from mixing. The simplicity of the setup meant telegraphic networks in the US and the UK used the gravity cell. Workers could spot when the crystals or zinc were exhausted and top up the cell. The only problem was that you could not move it once set up or the liquids would mix.
Teaching goal
Zinc ions dissolve as you dip the anode into the solution and the metal ions migrate downwards. To provide a supply of copper ions for the cathode we add the crystals to create a concentrated solution at the bottom of the beaker. Electrons travel through the wires from the zinc to the copper.
Even before the addition of copper sulfate, unless using freshly boiled deionised water, the buzzer will probably chirp. This is due to the dissolved oxygen in the water (see Nailing corrosion). The standard electrode potentials allow either oxygenated water or copper to couple with zinc with an electromotive force, EMF, in the region of 1 V (deviations result from the fact that the concentrations are non-standard):
| Half-equation | EMF (V) |
|---|---|
| ½O2(g) + H2O(l) + 2e-⇌ 2OH-(aq) | +0.40 |
| Cu2+(aq) + 2e-⇌ Cu(s) | +0.34 |
| Zn2+(aq) + 2e-⇌ Zn(s) | -0.76 |
I hope your students are as enthusiastic about this experiment as mine.
Even before the addition of copper sulfate, unless using freshly boiled deionised water, the buzzer will probably chirp. This is due to the dissolved oxygen in the water; watch the ‘Nailing corrosion’ video and read the article for an explanation (rsc.li/4h3EEND). The standard electrode potentials allow either oxygenated water or copper to couple with zinc with an electromotive force, EMF, in the region of 1 V (deviations result from the fact that the concentrations are non-standard):
½O2(g) + H2O(l) + 2e- ⇌ 2OH-(aq) +0.40 V
Cu2+(aq) + 2e- ⇌ Cu(s) +0.34 V
Zn2+(aq) + 2e- ⇌ Zn(s) –0.76 V
I hope your students are as enthusiastic about this experiment as mine.
More resources
- Spiral your curriculum for electrochemistry success and use this tried-and-tested practical sequence to build learners’ understanding of this tricky topic from 11–14 to post-16.
- Use number grids – an effective visual aid you can count on – to teach electrochemical cells.
- Read this CPD article, packed with classroom tips, activities and ideas to combat misconceptions, to plan your electrochemistry lessons.
- Watch the 16–18 electrochemical cells practical video and set microscale experiments for your 14–16 classes too.
- Find ways to overcome electrochemistry miconceptions in this article and download the resource to check learners’ understanding of how to identify the anode and cathode.
Declan Fleming
Want more? Find a sequence of electrochemistry practicals from p10 and a photo of a historic cell on p20.
Downloads
Gravity cell technician notes
Handout | PDF, Size 0.22 mbGravity cell technician notes
Editable handout | Word, Size 0.5 mb























2 readers' comments