Get started
Watch a demonstration of this experiment and download the technician notes from the Education in Chemistry website: rsc.li/4isOw3O
For much of modern history, when humans have sought to mop up liquids, we’ve looked to cellulose to do the job. Cast your eyes around your bathroom for the evidence – you’ll find it in toilet paper and sponges. But in the second half of the 20th century, material chemists developed so-called super slurpers. These polymers were able to absorb hundreds of times their own mass in water, retain it for longer and stop the material becoming too bulky.
Now, you can find superabsorbent materials like sodium polyacrylate all around you, in food packaging, surgical dressings and of course nappies, to make liquids ‘disappear’. With this experiment you can demonstrate how chemists can modify the structure of a material, turning one invention into many. Use it when teaching polymers and their properties to 14–16 year-old learners.
Kit
- Sodium polyacrylate powder, approx 3 g (irritating to eyes and skin; see tips)
- Fake snow/instant snow powder, approx 2 g (optional)
- Six disposable drinking cups: three opaque and three transparent
- 100 cm3 tap water
- Salt, approx 2 g
- Teaspoons × 3
Health, safety and disposal
- Although its gels are low hazard, powdered sodium polyacrylate is irritating to the skin and a serious eye irritant. Wear eye protection when transferring the powder and avoid raising dust.
- Dispose of any gel residue with normal waste.
Health, safety and disposal
- Although its gels are low hazard, powdered sodium polyacrylate is irritating to the skin and a serious eye irritant. Wear eye protection when transferring the powder and avoid raising dust.
- Dispose of any gel residue with normal waste.
Preparation
Wear eye protection. Place half a teaspoon (approx 1.5 g) of solid sodium polyacrylate in an opaque disposable cup. Stack this cup between two others ready to show the class. Measure out half a cup (approx 100 cm3) of tap water.
In front of the class
Ensure the audience is far enough away to miss the powder in the middle cup. Lay out three cups in a row and announce you’ll pour water into the middle one. Pour in the water and ask the audience to track the water as you shuffle the cups.
Let a member of the group guess which cup contains the water. When you upend the cup, it appears empty. Repeat with another audience member – again, no water. Finally, upend the last cup, even over someone’s head because you know (and can afterwards show) that it’s empty. Stir a teaspoon of table salt into all three cups and set aside.
Repeat the experiment with transparent cups to reveal the secret: your audience will see the powder and see the gel forming as the water meets it.
Then, revisit the first set of cups. Two contain only salt, while slushy liquid pours out of the third. Optionally, repeat with instant snow and show the resulting fluffy crystals that form instead of a gel.
Tips
- Buy sodium polyacrylate powder from educational suppliers (it may also be called slush powder, water gel or instant solid powder) or extract it from nappies by cutting into the lining and shaking out the powder. Do so in a closed box like an ice cream tub to avoid raising dust.
- If you’re not in a lab, use tap water and take a swig of the liquid beforehand to emphasise the surprising effect. See step six of Magical demonstrations for more on why you should make demos convincing.
Tips
- Buy sodium polyacrylate powder from educational suppliers (it may also be called slush powder, water gel or instant solid powder) or extract it from nappies by cutting into the lining and shaking out the powder. Do so in a closed box like an ice cream tub to avoid raising dust.
- If you’re not in a lab, use tap water and take a swig of the liquid beforehand to emphasise the surprising effect. See step six of Magical demonstrations for more on why you should make demos convincing (rsc.li/4hj4sVm).
Teaching goal
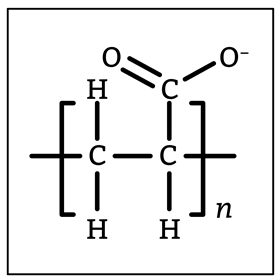
Sodium polyacrylate consists of a poly(ethene) backbone with anionic carboxylate side groups. Sodium ions balance the negative charge. The chains are crosslinked to some degree, which leads to a porous membrane at the surface of the polymer beads. It also prevents water completely separating the chains to dissolve them. Water can diffuse into this structure down the concentration gradient while sodium ions, in turn, diffuse out. Left to its own devices the polymer chain will twist and coil to minimise strain, but water will form hydrogen bonds between different parts of the polymer causing the material to swell. Use this demonstration to build on students’ prior knowledge and make links to everyday products containing the polymer.
Tap water is sufficient to make this demonstration work (the material will absorb approximately 300 times its mass in water) but minimising the ion concentration in the solution will increase the absorbency, so deionised water works even better. Easily show the reverse effect by adding salt, which increases the sodium concentration around the polymer, pulling the water back out.
Small tweaks to the material can help chemists fine tune it to their needs. In the case of instant snow, the material is largely the same but with fewer anionic carboxylates and tight clusters of highly crosslinked chains. This means that it can absorb less water, leading to a drier, fluffier hydrated form. Time for a snow day in May.
More resources
- Illustrate polymer properties with a self-siphoning solution, including a video, technician notes and teaching tips.
- Model large molecules with this activity suitable for 11–14 learners and make condensation polymerisation simpler with foldable paper prompts.
- Download this infographic poster, fact sheet and resource for everything you need to teach addition polymerisation.
- Mould your students’ understanding of polymers with these misconception busters and teaching ideas at 14–16 and post-16.
Declan Fleming
Downloads
Superabsorbent polymers technician notes
Handout | PDF, Size 0.13 mbSuperabsorbent polymers technician notes
Editable handout | Word, Size 0.47 mb























No comments yet