Cell chemistries of batteries have developed as their applications have grown, from flashlamps and radios ca 70 years ago to a host of consumer products today - watches, mobile phones and laptop PCs - and bigger standby batteries for emergency use in hospitals, department stores, telephone exchanges etc. Within each of these areas there are the demands for longer lasting, cheaper and environmentally benign batteries and cells.
-
An excursion through the cell chemistries of primary and secondary batteries, from Daniell's 1836 design to today's microbatteries
- The lithium-based rechargeable cell is the size of a grain of rice and has a 10-year life span

Count Alessandro Volta of Italy described the first battery in a paper to the Royal Society in 1799. His battery, which became known as Volta's pile, comprised alternating discs of zinc and copper with pieces of cardboard soaked in brine between the metals. From then on several other cell chemistries were introduced and manufactured batteries soon followed. Notably, the Daniell cell was invented in 1836 by British chemist John Frederic Daniell (see Box 1). In the early days the chemistry was confined to aqueous reactions but as electrochemistry developed interest turned to non-aqueous reactions as an alternative. In this article we take a look at the range of cell chemistries exploited in primary and secondary batteries on offer, from the tiny batteries for medical implants to the 100,000,000 watt-hour molten-sodium batteries which are used in power stations for load levelling.
Primary cells
These are the disposable cells - they are discharged once and discarded - that for over a century powered small and portable equipment. Nowadays as we become more waste conscious we find that many of these cells are giving way to rechargeable (secondary) cells and batteries.
The most common primary cells are based on the zinc-manganese dioxide couple: either zinc-carbon cells or alkaline manganese cells. For a short period some manufacturers offered mercury cells, which were replaced by zinc-air batteries, and some companies now produce 3V lithium-MnO2 cells.
Zinc-carbon
This cell became commercially available in the late 1800s and was a dry cell version of the original wet Leclanché cell. (The latter was made up of a conducting solution (electrolyte) of ammonium chloride with a negative terminal of zinc and a positive terminal of manganese dioxide.) Replacing the liquid electrolyte with a gel and then sealing the whole cell made it suitable for domestic applications where portability was a key feature. These cells were developed and marketed by Ever Ready for use in radios and torches and are still popular today, though Ever Ready has now changed to Energizer and many other makes have become available.
The dry cell zinc-carbon chemistry performs well in applications where there is intermittent use such as flashlights but performance is not so good in devices that put a heavy drain upon the cell. In such applications polarisation and loss of output results. However, the cell chemistry recovers when left idle for a while - the well known case of the dead battery that comes back to life. Polarisation is caused by the products of the electrode reactions building up on the electrode surface and preventing new reactants arriving. On standing, diffusion occurs and new reactants reach the electrode as the products disperse.
Anode: Zn metal
Cathode: MnO2 powder with graphite powder for electrical conduction
Electrolyte: NH4 Cl(aq) and/or ZnCl2 (aq)
Reaction: Zn + 2MnO2 + 2NH4 Cl → 2MnOOH + Zn(NH4)2 Cl2E = 1.5V
In earlier cells the zinc doubled as the outer can which tended to leak as the cell ran down and the zinc passed into solution.
Alkaline-manganese

These batteries developed from the zinc-carbon cell and became available for domestic use in the 1960s and quickly gained in popularity because they were less prone to polarisation, had greater capacity and were less likely to leak.
Here the anode is powdered zinc, which provides a greater reactive surface area and thus more power. The electrolyte is an alkali in contrast to the previous cell which was acidic.
Anode: Zn metal powder
Cathode: MnO2 powder with graphite powder for electrical conduction
Electrolyte: KOH(aq)
Reaction: Zn + 2MnO2→ ZnO + 2Mn2 O3E = 1.5V
Development of these batteries continues and recently Panasonic introduced a vacuum forming process to compact manganese dioxide and graphite powders, which the company claims gives improved capacity.
Mercury cells
These were not on the domestic market for long, being phased out in 1990s by most governments for environmental reasons. This is not surprising if we consider that a mercury cell could contain as much as 45 per cent mercury (II) oxide and, being a disposable domestic item, was destined to end up in landfill or be incinerated. Either way, it represented a worrying input of a neurotoxin into the environment. (It will be some years before mercury from remaining batteries has completely worked its way down the path from use to disposal and into the environment.)
In their short stay the mercury cells did an excellent job because they offered good capacity, long storage life and a stable voltage of 1.35V. This stability is in contrast to other cells where the voltage depends on the state of discharge. These properties made them ideal for cameras with a flash and watches with a backlight. They were also used for small electrical devices, including toys. In fact, only a couple of years ago a free Spider-Man toy containing a mercury battery was put into special packs of Rice Krispies in the US, much to the concern of health authorities.
Anode: Zn metal
Cathode: HgO
Electrolyte: KOH(aq)
Reaction: Zn + HgO → ZnO + Hg E = 1.35V
Zinc-air
A potential replacement to mercury cells came with another alkaline cell, the zinc-air cell, which uses air as its cathode. The air enters through a hole in the cell and oxygen is adsorbed onto a graphite surface where it undergoes reduction to give oxide ions.
Anode: Zn metal powder
Cathode: O2
Electrolyte: KOH(aq)
Reaction: 2Zn + O2 + 2H2 O → 2Zn(OH)2E = 1.65 V
Simultaneously, as the oxygen is being reduced, oxidation takes place at the zinc electrode, providing electrons. This cell has the advantage of being lighter than cells with other types of cathode chemistry. A disadvantage, however, is that carbon dioxide from the air reacts with the potassium hydroxide electrolyte, converting it to the carbonate, and so an absorbing system needs to be used, adding to the weight and taking away some of the advantage of an air electrode. Similar to the zinc-air cell is the aluminium-air cell.
Lithium cells
Lithium is the lightest metal, has an electrochemical potential of over 3V, and low toxicity. These factors make it an attractive prospect for batteries. However, being a Group I metal, lithium is highly reactive and therefore it is necessary to use a non-aqueous electrolyte. Lithium batteries generally have lithium metal (or alloy) as the anode but show an extensive range of chemistries for the cathode and the electrolyte.
Electrolytes can be either an organic liquid or a solid polymer - each with a dissolved lithium salt to make it conducting - or a fused lithium salt. A commonly used cathode is solid carbon monofluoride (a fluorine-graphite matrix). Some also have solid electrolytes, such as the lithium-iodine cell which has a very long life at low rates of discharge and is used in pacemakers. In this cell the cathode is a polymer into which iodine is absorbed.
Currently, the most popular lithium primary cell is the solid cathode type, comprising powdered manganese dioxide and graphite - not unlike the one used in the previous aqueous cells, along with a liquid electrolyte.
Anode: Li metal (or alloy) foil
Cathode: MnO2 powder with graphite powder for electrical conduction
Electrolyte: Li salt dissolved in aprotic solvent
Reaction: Li + Mn(IV) → Mn(III) + Li+E = 3V
These lithium cells are available as cylindrical cells, or as button cells. They have the added advantage of operating over a wide temperature range, from -40°C to +60°C.
There are numerous other examples of primary cell chemistry, including magnesium-manganese dioxide, a magnesium version of the alkaline-manganese type; and silver-silver oxide.
Secondary cells

For a rechargeable cell, or more commonly a battery of cells, to be successful the cell must be capable of many discharge and recharge cycles. For example an electric vehicle relying upon a secondary battery as its power source must be capable of being recharged a thousand times or so, before the battery is worn out. To achieve this, the chemistry must be reversible, otherwise there would be loss of capacity on each recharge cycle which would eventually render the cell useless. And so a rechargeable battery must have a long cycle life.
Traditionally rechargeable cells were used for high power applications such as engine cranking, electric vehicles and emergency lighting but are now making a big contribution in the domestic market and small rechargeables are a part of everyday life. Furthermore, this is an area which will grow as more portable electronic devices become available and as people want to move away from the throwaway batteries.
Lead-acid
The lead-acid battery, invented by Gaston Plant? in 1859, is the most widely used rechargeable battery. Over the years improvements have been made as new materials have become available. But though the electrode structure and container materials have changed, the basic electrode chemistry used is the same as it was over a century ago.
Anode: Pb metal as spongy powder
Cathode: PbO2
Electrolyte: H2 SO4 (aq)
Discharge reaction:
Pb + PbO2 + 2H2 SO → 2PbSO4 + 2H2 O E = 2V
The spongy lead is packed into cadmium-lead alloy grids and the lead dioxide pasted onto alloy plates. Recharging simply reverses the discharge reaction but is accompanied by the production of hydrogen and oxygen, owing to the electrolysis of water. Early batteries had therefore to be ventilated to allow the gas to escape and avoid pressure build up and the risk of explosion. The battery's electrolyte level had to be periodically topped up with demineralised water.
In the 1970s sealed batteries with pressure release valves became available. These systems contained a catalyst to convert the hydrogen and oxygen back to water. Later improvements included the use of a calcium-lead alloy as the electrode grid material in place of cadmium-lead alloys. Although these batteries are known as calcium batteries this is misleading because calcium plays no part in the cell chemistry.
Calcium-lead alloy batteries are less prone to self-discharge and are highly effective in providing a short burst of high current, which makes them suitable for use in cars for engine cranking. They are, however, unsuitable for prolonged discharge as required in electric vehicles or in emergency lighting facilities.
The traditional cadmium-lead alloy batteries do not tolerate deep discharge if they are not recharged soon after reaching this state. If left, the lead sulfate discharge product becomes stable and resists all efforts at recharging; the battery is said to be sulfated and is dead.
Nickel-cadmium
Despite the success of the lead-acid batteries, one major disadvantage is their weight and the aim of much research in this area has been to produce lighter versions. Of the different chemistries considered, nickel-iron (NIFE) and nickel-cadmium (NICAD) alkaline batteries had some commercial success at the end of the 19th century. Later, the NICAD battery enjoyed more success because it was less prone to corrosion, even challenging the lead-acid battery in applications such as emergency lighting despite its higher price. NICAD chemistry is now used for small rechargeable batteries for domestic use as well as heavier applications such as electric vehicles.
Anode: Cd metal
Cathode: Ni(OH)2
Electrolyte: KOH(aq)
Discharge reaction:
Cd + 2NiOOH + 4H2 O → Cd(OH)2 + 2Ni(OH)2.H2 O E = 1.3V
Nickel-metal hydride
The NICAD chemistry provided the basis upon which the nickel-metal hydride cell was developed. Again, nickel chemistry finds use in nickel-metal hydride (NiMH) rechargeables. As with the NICAD, these batteries are for small domestic use and for heavier applications such as electric vehicles. The NiMH cell stores almost twice the energy as the NICAD, a factor that has made them very popular.
Anode: MH where M = Alloy such as LaNi5
Cathode: NiOOH
Electrolyte: KOH(aq)
Discharge reaction: NiOOH + MH → Ni(OH)2 + M E = 1.35V
This cell came about when the hydrogen absorptive properties of certain metal alloys was discovered. In these alloys some of the component metals absorb hydrogen exothermically whereas others do so endothermically. By having the correct balance of each type in the alloy, hydrogen absorption and release may proceed with no net change in temperature which is desirable for cells. One of these alloys, LaNi5 can store six hydrogen atoms, LaNi5 H6.
Lithium ion
Metallic lithium, which is used in lithium primary cells, is unsuitable for rechargeable cells owing to dendritic crystal growth of the metal in the recharge phase which can damage the cell. Instead lithiated graphite is used as the anode.
Anode: Lithiated graphite (LiC6)
Cathode: LiCoO2
Electrolyte: LiPF6 in aprotic solvent
Overall reaction: Li 1-x CoO2 + CLix→ LiCoO2 + C E = 3.7V
Lithium atoms in the lithiated graphite are intercalated between the hexagonal layer molecular structure of the graphite and are free to move around. On discharge these atoms migrate from the graphite to the lithium cobalt oxide. This technology was developed by the British company AEA Technology and commercialised by Sony under licence from AEA. Currently Quallion in the US uses this technology in microbatteries (the size of a grain of rice) in medical implants for neurological disorders. The batteries have a 10-year life.
Molten sodium cells
Another Group I metal to have found use in batteries is sodium with the sodium-sulfur cell being an example. This cell is unusual in that it has liquid electrodes, molten sodium and molten sulfur, with a solid electrolyte. The system works at 350°C. With sodium and sulfur separated by only a couple of millimetres of ceramic, at this temperature the cell presented some tough design problems to avoid explosive reactions.
Sodium-sulfur batteries are being used for load levelling (100,000,000 watt-hours) in the electricity generating industry and to power electric buses, trains and tractors.
Anode: Na(l)
Cathode: S(l) with carbon fibre conducting matrix
Electrolyte: β-Al2 O3 ceramic
Overall reaction: 2Na + 3S → Na2 S3 and polysulfides E = 2.076V
β-alumina comprises sodium ions in an aluminium oxide lattice. During discharge the sodium anode releases sodium ions which migrate through the β-alumina to the liquid sulfur. These positive ions react with the sulfur to form sodium sulfide and polysulfides. A carbon fibre matrix acts as a conductor to carry electrons into the sulfide/sulfur melt. Recharging reverses the process.
The battery's temperature must be maintained and so its construction has to include heating elements. Sodium sulfur chemistry started at Ford Motors in the US but much of the successful development work was done in the UK at British Rail Research and at Chloride Power in the 1970s.
The sodium-nickel chloride cell also uses β-alumina as the electrolyte. This cell operates at 300ºC. Silver-zinc technology is now attracting interest for rechargeable batteries.
Tony Hargreaves is a science writer and a part-time lecturer in applied chemistry at Calderdale College of Further Education, Halifax.
Further Reading
- C. A. Vincnet and B. Scrosati, Modern batteries: an introduction to electrochemical powersources. New York: John Wiley, 1997.
- R. M. Dell and D. A. J. Rand, Understanding batteries. Cambridge: RSC, 2001.
- K. Othmer, Encyclopaedia of chemical technology, 4th edn. New York: John Wiley, 1992.
- T. R. Crompton, Battery reference book, 3rd edn. Oxford: Newnes, 2000.
- T. Hargreaves, Chem. & Indy, 5 Sept 2005
Related Links
AEA group
AEA is a leading international company specialising in consultancy, policy support and programme management for policy implementation
Energizer
Energizer is a world leader in battery technology
Duracell
Batteries designed to give you maximum power
Varta
Varta manufacture a range of automative and consumer batteries and microbatteries
Panasonic
Panasonic manufactures a range of batteries for industrial and consumer applications
Rayovac
Panasonic manufactures a range of batteries for industrial and consumer applications
Box 1 Chemistry of the Daniell cell
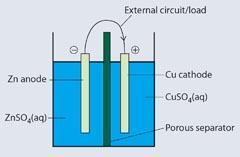
When the circuit is complete, electrons flow from the zinc to the copper, owing to the electrical potential. Conventional current flows from the positive (anode) to the negative (cathode): this is the opposite to the direction of electron flow.
The porous separator prevents bulk mixing of the electrolytes but allows aqueous ions to pass through to maintain the ionic balance.
Anode: Zn(s) → Zn2+ (aq) + 2e-
Cathode: Cu2+ (aq) + 2e-→ Cu(s)
For a copper-zinc cell under standard conditions (25°C, 1 mol dm-3, 1 atmosphere) the cell voltage may be calculated from the oxidation and reduction half reactions.
Zn(s) ½ Zn2+ | Cu2+ (aq) ½ Cu(s)
E° Zn2+ + 2e-→ Zn E° = -0.76V
E° Cu2+ + 2e-→ Cu E° = +0.34V
E° for the cell = +0.34 - (-0.76) = 1.10V








No comments yet