The Lasers for Science Facility in Harwell, UK, lets chemists see and manipulate chemical and biological systems. This research has a direct impact on our everyday lives.
-
Research at the CLF has developed techniques using lasers to detect explosives and bone disease
-
Optical tweezers can help scientists to understand the chemistry of clouds to give us better models of our climate
As well as developing and maintaining world-leading laser equipment for use by research teams throughout the UK, scientists at the Science and Technology Facilities Council's (STFC) Central Laser Facility (CLF)1 are pioneering and implementing the latest techniques in order to enhance the technology.
Lasers are extremely versatile and this is reflected in the lasers available at the CLF, which offer a vast choice of power, wavelength and pulse frequency to satisfy a plethora of research objectives.
For example, high power lasers can be focused to initiate nuclear fusion or recreate the extremely high temperatures and pressures found inside stars.
However, lasers are rarely just pointed at a target. Ultra-short pulsed lasers can be used to track transient changes in atoms and molecules, or the light can be manipulated in some way. For example, lasers have been used to cool matter to the lowest temperature recorded in the known universe (achieving a universal record, not just a mere world record). As we shall see, they can also be used as 'optical tweezers' to pick up and manipulate objects as small as microorganisms.
The combination of design flexibility and expert operation means that CLF lasers are very much more productive than they would be if each was limited to one specific task at a particular institution.
A solution looking for a problem
When lasers were invented in 1960, they were called 'a solution looking for a problem'. The tabloids ran stories about 'death rays', which might have inspired the scene in Goldfinger, the 1964 James Bond film, where the hero is strapped, spreadeagled, to a metal table while a laser cuts it in half (fig 1).
Lasers are now ubiquitous, with compact disk players and eye surgery vying as the most familiar applications. They have since generated billions of pounds sterling worth of business and at least 10 Nobel laureates can thank lasers for the breakthroughs they achieved.
How do lasers work?
Each chemical element has a unique set of electronic energy levels, sometimes known as a spectral fingerprint, which is the basis for spectroscopy - allowing scientists to use remote sensing to work out the composition of a star, for example.
The absorption of a photon with exactly the correct energy (corresponding to the difference between energy levels) will promote an electron to a higher energy level, exciting the atom. When the electron drops back down to a lower energy level, a photon corresponding to the difference in energy is emitted, but this process is spontaneous - taking place at a random time and with the photon going off in a random direction. This leads to the familiar absorption and emission spectra.
In stark contrast, laser light is notable for its high degree of spatial and temporal coherence. Laser light is highly monochromatic, which literally means 'one colour': all the photons are identical. They possess the same wavelength and hence the same frequency and energy. They are all in phase - they all march in step - and the resulting 'pencil beam' can be focused to a tiny spot, achieving a very high irradiance.
Einstein laid down the principles for a laser in his 1917 paper, On the quantum theory of radiation. However the first functioning device wasn't in operation until 1960, when it was built by Theodore Maiman.
The word laser started life as an acronym for light amplification by stimulated emission of radiation but it has since entered English as a noun in its own right. They key words are stimulated and amplification.
Photon emission can be stimulated by a passing photon that possesses the correct energy - corresponding to the difference in atomic energy levels. Both incident and emitted photons are identical; they possess the same energy and move off in phase (in step) in the same direction. For amplification to take place, we need something called a population inversion, which is perhaps best explained using a common example (see box).
In a helium-neon laser an electric field provides the energy used to 'pump' helium atoms to a metastable (ie relatively long-lived) excited state of 20.61 eV (fig 2). Helium atoms collide with neon atoms, exciting them to a metastable state of 20.66 eV - the extra 0.05 eV is provided by the kinetic energy of the colliding helium.
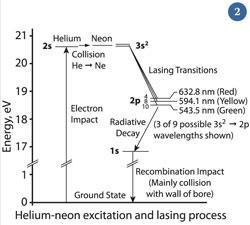
The neon atoms now have more atoms in their metastable state than the first excited state (18.70 eV). A photon whose energy corresponds to the difference between the metastable and the first excited states (ie 1.96 eV) stimulates emission.
Mirrors at either end of the laser tube reflect the identical photons so that they sweep back and forward through the cavity, press-ganging clones of themselves by stimulating all those in the metastable state of neon to join them. Typically one of the two mirrors, the output coupler, is partially transparent and the laser beam leaves through this.
Lasers at CLF

The CLF's two high-power lasers, Vulcan and Astra Gemini, are both used for plasma physics. The light they produce can be focused to rip matter apart, creating a gas of ions and electrons.
Vulcan has a footprint bigger than six tennis courts (fig 3) and can deliver up to 2.6 kJ of laser energy. It makes major contributions to Inertial Confinement Fusion (ICF), critical to the development of fusion energy.
A pinhead-sized pellet containing the heavy isotopes of deuterium (D) and tritium (T) is placed at the intersection of several laser pulses, compressing and heating the D-T mixture and 'igniting' nuclear fusion. The mass that 'goes missing' when deuterium and tritium are fused into helium-4 plus one neutron turns into pure energy, according to Einstein's famous formula E = mc2.
Once fusion is mastered, STFC and the scientists working at the Rutherford Appleton Laboratory (RAL) can take credit for their part in providing a clean source of energy, with little waste and certainly no greenhouse gasses. It will also provide energy security by reducing or removing our dependence on imported fuels and may play a part in reducing international conflict.

Astra Gemini drives similar areas of plasma research but its design allows it to fire shots much more quickly than Vulcan can - up to three per minute compared with Vulcan's few per hour. Astra Gemini can fire pulses with intensities of up to 10 000 times greater than the centre of the sun. This makes Gemini the most intense user facility in the world (fig 4).
Artemis is another powerful laser but, instead of creating plasmas, it is set up to allow fast-moving processes such as chemical reactions to be studied in much more detail. This is because pulses from Artemis can be much shorter than synchrotron radiation - down to a few femtoseconds.
To gain an insight into how this can be used, imagine recording each pulse as a photograph and playing back a series of images to create a movie. The laser light from Artemis can be tuned from the infrared (20 000 nm) to the soft X-ray (15 nm), making it a very versatile machine.
In contrast, the other laser systems in the CLF are precise tools for studying delicate systems and come under the umbrella of the Lasers for Science Facility (LSF), the focus of the rest of this article.
Located in the Research Complex at Harwell, the LSF promotes interdisciplinary research across chemistry and biological science. The LSF can be further sub-divided into several groups who collaborate closely. The Molecular Structure and Dynamics (MSD) Facility houses the ULTRA laser, which is one of the world's best instruments for infrared-visible-ultraviolet ultra-fast spectroscopy and the scientists using it have an international reputation in the dynamics field. Research using optical tweezers is done here, working closely with the Functional Biosystems Imaging (FBI) group.
The FBI group houses the OCTOPUS cluster of lasers, which enable real time imaging of complex physical and biological systems, integrating a very wide range of techniques.
With Raman spectroscopy, the shift in wavelength of the scattered light reveals molecular structure and provides a unique spectral signature.
A photon of laser light can excite a molecule. If the molecule relaxes back to a different state, the emitted photon will have a different frequency (energy) from the one that was absorbed and the shift in energy can be used to infer the different vibrational and rotational states of a molecule and hence give away the identity of the molecule itself.
Case study 1 - What lies beneath?
Spectroscopy can reveal chemical composition. Shine light at a transparent substance or a dissolved sample and the composition of it can be revealed by the absorption spectra. Likewise, the reflected light from an opaque substance can betray its chemical composition.
But what if you wanted to know what lies beneath the surface of a milky liquid or even a solid? A technique called spatially offset Raman spectroscopy (SORS)2 is being developed that will do just that. Close collaboration with STFC Innovations Ltd. is commercialising this technique through the spin-out company Cobalt Light Systems.3
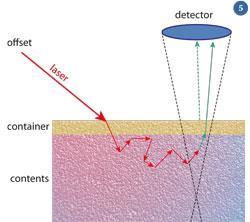
The SORS offset is introduced by laterally moving the laser beam on the sample surface. When a ray of laser light is shone at an opaque object most will be reflected and this will be seen as a laser spot on the surface. Some laser light will pass into the bulk; that scattered back along the line of incidence will be 'outshone' by backscattering at the surface. However, photons that penetrate several millimetres into the bulk are also scattered and diffuse sideways, as shown by the 'random walk' in fig 5, and reappear at the surface outside the incident laser spot.

Therefore, Raman photons detected in the vicinity of the laser illumination are strongly biased towards the surface signals and collection away from the laser illumination area favours signals emanating from deeper in the sample.
Experiments have shown that SORS can detect hydrogen peroxide, a potential explosive, even when concealed in a typical plastic cosmetics bottle. If SORS could be deployed at an airport check-in, (fig 6) perhaps the ban on carrying liquids in hand luggage onto aeroplanes might be lifted.
In collaboration with University College London, research is ongoing to find out if SORS could be used to detect bone disease through soft tissue, while research with Gloucestershire Royal Hospital will establish if breast cancer can be diagnosed noninvasively by looking for signs of calcification in breast tissue.
Case study 2 - Lab in a cell
Imagine being prescribed a drug that is designed specifically for you and has no side effects. This is the vision of Lab in a cell.
Until now, disease control has involved identifying the protein (eg enzyme or receptor) associated with a particular disease and designing a drug molecule that locks onto the protein to block the process. However, the protein may also be responsible for beneficial biochemistry so the drug can cause side effects.
While unravelling all the possible molecular interactions in a cell might seem a hopeless task, physicists, computer scientists and biologists are working together to develop a 'systems' approach to understanding cell biology. Researchers at the CLF and King's College London are working on one piece of this jigsaw.
They 'colour-code' each molecule with a different fluorescent dye so that it glows when illuminated by laser light of the right wavelength.
Using the facility's suite of advanced laser microscopes, they image different sets of molecules simultaneously with the aim of pinning down the interactions between them. Molecular interactions cause a change in fluorescence, which can be observed. Subtle changes in shape can betray cell signalling and this can be detected by changes in the polarisation of laser light.
Case study 3 - Optical tweezers
Lasers can be used to hold tiny objects in 'optical tweezers' for closer inspection or even to measure forces on the molecular scale.4 These 'tweezers' can also be used to measure the force required to unzip the DNA double helix or how hard a virus has to work to force its way into a cell.
How are 'optical tweezers' created?
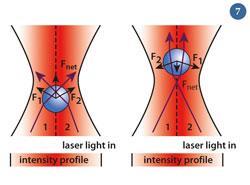
Sending a laser beam through the objective lens of a microscope focuses the beam. A micrometer-sized dielectric bead is attracted to the centre of the beam waist, the narrowest point of the focused beam, where the electric field is strongest.
It is perhaps easiest to understand why the bead stays in the beam waist by using a ray optics approach (fig 7). The light ray is refracted by the bead and so the photons change direction (significant because momentum and force are vectors, whose value changes with direction as well as magnitude). So refraction equates to a change in momentum for the photons and hence they experience a force. According to Newton's Third Law of Motion, there should be equal and opposite forces on the bead (labelled Fnet), always pushing it towards the centre of the waist.
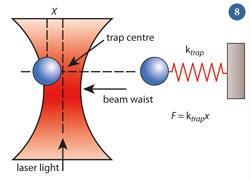
The optical trap created acts like a simple spring that obeys Hooke's law F = kx , where F is the applied force, k is the 'spring constant' and x is the extension (fig 8). A force applied to the bead will push or pull it away from the laser focus, its equilibrium position. A bigger force is required to move the bead further from its equilibrium position. Conversely, measuring how far the object moves and multiplying the result by the 'spring constant' of the tweezers determines the size of the force.
Cloud chemistry
Optical tweezers can be used to study cloud chemistry, one of the biggest uncertainties in computer models of the climate.
By spraying mist into the laser beam, one of the droplets finds its way into the waist, where it becomes trapped. Raman spectroscopy can be used to follow changes in chemical composition of the droplet surface over time in response to exposure to ozone or organic molecules that simulate seawater.
It might be the ideal test-bed to study the role of DMS (dimethyl sulfide) as condensation nuclei, something first mooted by James Lovelock as evidence for his Gaia hypothesis.
A solution looking for new problems
Half a century ago, lasers were a 'solution looking for a problem'. Now they are at the forefront of research that will change our lives beyond recognition and may yet solve the energy crisis. The STFC's Central Laser Facility is world class and its strength lies in the interdisciplinary teams that are built around each laser.
The work being undertaken by scientists at RAL could fill several volumes of Education in Chemistry and readers are encouraged to follow the links to the resources provided by STFC, which are given given below.
Mike Follows teaches at King Edward's School, Birmingham, and is writing articles as Science Teacher Eminus for the Rutherford Appleton Laboratory.
Resources
A wide range of supporting teaching resources are available from the STFC Public and Schools website.
This includes the Naked Scientists Podcasts, which are supported by the STFC. The Naked Scientists visited Artemis - the Superfast XUV Laser.
It is also worth searching for the excellent Backstage Science videos, which describe the various instruments and some of the research being undertaken.
Related Links
Hear the Naked Scientists visit the super fast Artemis laser
References
2. http://bit.ly/RamanPapers. This includes the paper Spatially offset raman spectroscopy (SORS) for liquidscreening at http://bit.ly/SORSliquids (pdf).
3. www.cobaltlight.com
4. M R Pollard et al, New J. Phys., 2010, 12, 113056 (DOI: 10.1088/1367-2630/12/11/113056)
5. D T Clarke et al, Rev. Sci. Instrum., 2011, 82, 93705 (DOI: 10.1063/1.3635536)









No comments yet