Chemists from the Universities of Loughborough and Bristol have teamed up to take a research-based project into local schools. The project, which involves using Grätzel solar cells to convert sunlight into electricity, gives students the opportunity to be involved in cutting-edge, green chemistry research
- Project provides links to GCSE science and A-level chemistry specifications
- Grätzel cells - TiO2 coated with light-sensitive dye molecules, immersed in organic electrolyte - mimic photosynthesis

Three years ago we applied to the Engineering and Physical Sciences Research Council Partnerships for Public Engagement (EPSRC PPE) scheme for funding to get GCSE and AS/A2 students involved in a contemporary science research topic. We chose an area of research - Grätzel electrochemical solar cells - that is not only active in our own department of chemistry at Loughborough but is also multidisciplinary. Research in this area involves electrochemistry, material science, biological analogues, the fundamental physics of charge transfer, the synthesis of new dye molecules, and the socio-economic impacts of solar power.
Through this project, we reasoned, students would learn how the development of new commercial devices requires a broad science background. Further, we believed that if school students could engage in relevant, contemporary research and meet the researchers, they would go on to study science post-16 and eventually choose a science-based career. We teamed up with colleagues in the University of Bristol because we wanted to get as many schools as possible from the surrounding areas involved in the project.
Grätzel solar cells
The world's energy supplies rely heavily on non-renewable sources - coal, oil, and natural gas. All of these produce the environmentally damaging greenhouse gas, CO2, and all are finite, they will eventually run out. In contrast, renewable energy resources, such as solar, wind, waves/tides, biomass, hydrogen, and geothermal energy, are constantly replenished.
In this project we focus on solar energy. The energy of less than one hour of sunlight is equal to the total yearly human energy consumption. So if a fraction of the solar energy reaching the Earth could be harnessed, many energy supply and environmental problems associated with reliance on fossil fuels would be solved.

A variety of technologies has been developed to harness solar energy. For example, photovoltaic solar cells,1 typically based on monocrystalline silicon wafers, are efficient but expensive to manufacture, and therefore not attractive for large-scale commercial and industrial applications. At Loughborough we have been investigating Grätzel solar cells as a potentially cheaper alternative. Invented in 1991 by Michael Grätzel and coworkers at the Swiss Federal Institute of Technology, Lausanne, Grätzel cells comprise nanometre crystals of the semiconductor titanium dioxide (TiO2, anatase)2 coated with light-sensitive dye molecules such as bipyridyl ruthenium(II) complexes. The coated crystals are immersed in an iodide/tri-iodide organic liquid electrolyte. Since TiO2 is abundant, non-toxic and of low cost, these cells have the potential to be highly economical compared with the commercial silicon photovoltaic solar cells. Essentially Grätzel cells mimic natural photosynthesis.3
Mechanism of Grätzel cell
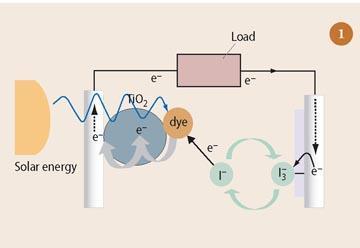
The mechanism for converting solar energy into electrical energy in a dye-sensitised solar cell is a five-step process (see Fig 1).
- Solar energy (hν as photons of light) causes electrons in the molecular orbitals within the adsorbed dye sensitiser (S) molecules to become photoexcited (S*):
S(adsorbed on TiO2) + hν → S*(adsorbed on TiO2) (i)
(The trapping of solar energy by a sensitiser molecule is analogous to the light-absorbing chlorophyll molecule found in Nature, which converts carbon dioxide and water to glucose and oxygen.)
-
The excited electrons escape from the dye molecules:
S*(adsorbed on TiO2) → S+(adsorbed on TiO2) + e- (ii)
-
The free electrons then move through the conduction band of TiO2, gather at the anode (the dyed TiO2 plate), and then start to flow as an electric current through the external load to the counter electrode.
-
The oxidised dye (S+) is reduced to the original form (S) by regaining electrons from the organic electrolyte solution that contains the iodide/tri-iodide redox system, with the iodide ions being oxidised (loss of electrons) to tri-iodide ions:
S+(adsorbed on TiO2) + 3/2I- → S(adsorbed on TiO2) + ½I3- (iii)
To restore the iodide ions, free electrons at the counter (graphite) electrode (which have travelled around the circuit) reduce the tri-iodide molecules back to their iodide state. The dye molecules are then ready for the next excitation/oxidation/reduction cycle.
Putting it all together
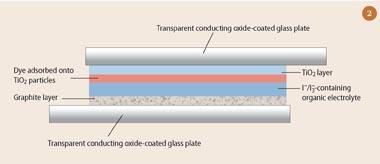
Grätzel cells comprise two transparent conducting oxide glass plates sandwiched together (Figs 2 and 3). Typically fluorine-doped indium oxide or tin-doped indium oxide are used. The thin oxide coating on one side of the glass makes the glass surface electrically conducting. The upper plate (the 'photoanode'), has the sensitising dye adsorbed onto a 10 μm layer of randomly-stacked nanoparticles (ca 20 nm in diameter) of TiO2.
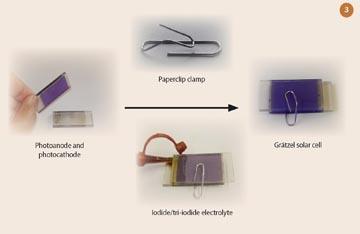
For research investigations, a thin film of platinum is chemically deposited onto the lower plate as a conductor and catalyst. However, for demonstrations, simply coating the lower plate with graphite from a soft pencil will suffice. The upper and lower plates are secured together with a paperclip 'clamp' (Fig 3). To finish the cell, a drop of organic electrolyte solution, which contains the iodide/tri-iodide redox couple, is applied between the plates.
Frontier research

Since the ground-breaking report of 1991,2 there has been an explosion of research activity across the world into 'Grätzel' solar cells,4-9 which convert sunlight into electricity with remarkable efficiency (>10 per cent in simulated AM-1.5 solar light).10
The technology relies on the fact that porous films have very high surface areas (at the molecular level). Only the first monolayer of adsorbed dye results in efficient electron ejection into the semiconductor, but the light-harvesting efficiency of a single dye monolayer is very small. In a porous film consisting of cubic-close packed, 15-20 nm-sized spheres in a 10 μm-thick layer, the effective surface area is enhanced ~1000-fold, thus making light absorption very efficient even though there is only a single dye monolayer on each particle.
Numerous research groups across the world are busy developing novel synthetic dyes with even better energy conversion efficiencies.
The outreach project

In the EPSRC Partnerships for Public Engagement project, our initial target was to involve students and their teachers in 10 schools in the local areas. Part of the funding allowed us to give each of the schools five commercial Grätzel solar cell kits, along with support material for teachers and students - a PowerPoint presentation on CD, our own manual How to make your own Grätzel solar cell, and investigations that could be done using the kits.
Teachers were also given the opportunity to attend a practical workshop and/or attend a presentation and practical demonstration and/or meet with the project research assistant (Dimple Patel) for a informal discussion. Numerous workshops and demonstrations have taken place, including contributions to University Experience days and outreach activities held at Loughborough University and elsewhere. In addition, Grätzel solar cell workshops for teachers and PGCE students have been held at both Loughborough and Bristol Universities. The PGCE students prepared written resources, which they subsequently used on their teacher training placements.
For schools-based demonstrations and investigations, use of the commercially available Grätzel Cell demonstration kits is most convenient. Each kit allows the students to prepare six (or 12) solar cells to power a small electronic calculator or sound 'chip'. The kits include 12 (or 24) conductive glass plates to make the 6 (or 12) 'sandwich' solar cells, crocodile clips with leads, nanoparticulate TiO2 powder paste mix, iodide/tri-iodide-containing organic electrolyte, a multimeter (for measuring electrical current, voltage and resistance), a small electronic calculator, a sound 'chip' and a pack of hibiscus tea leaves for preparing the dye solution. The cheaper kits provide the materials to prepare films of TiO2 onto the conductive glass plates, while the more expensive kits have them already prepared.
Experimental tips

To make the TiO2 plates a little time needs to be invested. Using a microscope slide, apply the TiO2 powder and paste mix as a colloidal suspension. Electronic contact between the TiO2 particles is produced by sintering at 450°C (30 minutes in an oven or furnace) or heating over a (blue flame) Bunsen burner. If you use a Bunsen burner, the sintering process should not take more than three minutes to complete. The white TiO2 coating will start to colour brown after approximately one minute and then gradually turn white again. At this point, the sintering process is complete. Conductive glass slides and TiO2 -coated conductive glass slides can be bought separately for additional solar cells or for schools which have access to the other kit components.
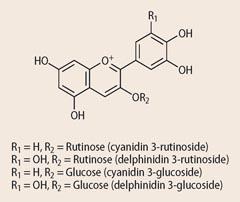
Various natural organic dyes can be adsorbed onto the TiO2 nanoparticles for use as the light-harvesting sensitiser molecule. The best natural dyes are the purple-red anthocyanins (1), found in blackberries, raspberries, beetroot, cranberries, cherries, hibiscus tea leaves, and blackcurrants. To extract the dye from blackberries or raspberries, add four berries to a mortar with 20 cm3 of warm water and crush the berries using a pestle. To prepare the hibiscus tea dye soak a few grams of the dried leaves in boiling water for a few minutes and decant the solution. Stain the TiO2 plates by immersing them into the dye solution for 10 minutes and then dry using a hair drier. The dyed TiO2 plates are re-usable, the dye can be removed by leaving the plates to soak in water for a few hours. The process is enhanced under exposure to uv light, taking advantage of the photocatalytic properties of TiO2.
Links to the secondary curricula
This project links to GCSE Science, as well as to AS and A2 chemistry specifications. For example, in the Edexcel GSCE Science 2006 specification there are several 'core' topics which could be supported by this project, including: topic 8 (Designer products), which covers 'nanotechnology and nanoparticles'; topic 9 (Producing and measuring electricity); and topic 10 (You're in charge), which covers 'electric power, solar power, efficiency of solar cells and renewable energy'.
In A-level chemistry relevant link topics include: the dye (transition metal complexes); the TiO2 substrate (materials chemistry); the mechanism of energy conversion (redox chemistry); light absorption (UV-visible spectroscopy); alternative energy (combustion and/or environmental chemistry); and chromatography (separation of dye molecules from foods). Furthermore, materials, electronic, electrical and photosynthesis aspects will include concepts learnt in A-level physics and biology. Thus, the circuits that are assembled to generate electrical energy and to measure electrical parameters and cell efficiencies can be studied in physics; the strategy of separating charge across an interface can be related to biological photosynthesis.
And finally...
Our initial target of 10 schools target was surpassed. We have already put kits into 35 schools, while a further cohort has been reached through several other initiatives, for example the Aimhigher Chemistry: the next generation project led by the Royal Society of Chemistry.
Plans for further work related to the project will include ongoing contact and support of teachers in all 35 schools as well as outreach to additional teachers and schools through the Teacher Education Unit at Loughborough University.
For more information on the use of these kits in schools please contact R.J.Mortimer@lboro.ac.uk, or D.R.Worrall@lboro.ac.uk at Loughborough University or T.G.Harrison@bristol.ac.uk. The Grätzel kits are available from Man Solar. All the support materials are available to view/download from website and include details of investigations at a number of different levels.
Professor Roger J. Mortimer and Dr David R. Worrall are lecturers in the department of chemistry at Loughborough University, Loughborough, Leicestershire LE11 3TU.
Acknowledgements
I thank Dimple Patel (Loughborough University), the project research assistant, and Tim Harrison (University of Bristol) and Jason Riley (Imperial College, formerly Bristol University) for their involvement in this project.
Also of interest
Chemistry: The Next Generation
Chemistry: The Next Generation
References
- J. Twidell and T. Weir, Renewable energy sources, 2nd edn, chap 7. London: Taylor and Francis, 2006.
- B. O'Regan and M. Grätzel, Nature (London), 1991, 353, 737.
- A. Kay and M. Grätzel, J. Phys. Chem., 1993, 97, 6272.
- A. Hagfeldt and M. Grätzel, Chem. Rev., 1995, 95, 49.
- M. Grätzel, Nature (London), 2001, 414, 338.
- M. Grätzel, J. Photochem. Photobiol., C, Photochem, Rev., 2003, 4, 145.
- M. Grätzel, Inorg. Chem., 2005, 44, 6841.
- Md. K. Nazeeruddin and M. Grätzel in Comprehensive coordination chemistry II: from biology to nanotechnology, J. A. McCleverty and T. J. Meyer (eds), Vol 9, pp 719-758, M. D. Ward (ed). Oxford: Elsevier, 2004.
- Md. K. Nazeeruddin et al, Coord. Chem. Rev., 2005,249, 1460.
- Md. K. Nazeeruddin et al, J. Photochem. Photobiol. A: chemistry, 2007, 185, 331.









No comments yet