It’s strange to think that no one knows exactly how smell works. Josh Howgego explains the chemistry behind the puzzle

In laboratories up and down the country there are chemists who have the misfortune to work with thiols. Some of these whiffy molecules have a stench so pungent, so disgusting, that they have been known to empty entire buildings when a few drops are spilled in the wrong place.
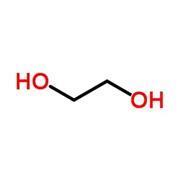
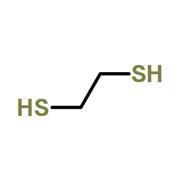
But why do thiols smell so bad? It doesn’t seem to make sense when you consider their structures (fig 1). Thiols are the sulfur analogues of alcohols – they look very similar, so what makes their odours different?
The answer is surprisingly complicated, and scientists are still arguing about it. It’s strange to think that smell remains a mysterious process.
You would think that vision – which we understand quite well – would be a much more complicated sense than smell. But actually our eyes only have to detect one thing: photons (albeit with different wavelengths). Whereas our noses are chemical sensors: capable of identifying thousands of different molecules.
Smells of the past
Smell has an emotional power over us that the other senses can’t emulate. The scent of cut grass on a sunny day can transport us back to memories of childhood, and we are beginning to understand why smell can be so evocative. The brain’s olfactory bulb – the first port of call for impulses from the nose – has a direct neural link to the limbic system, the region of the brain that processes memory and emotions. In contrast the major nerves from all the other senses are routed via the thalamus for pre-processing before the information is fully interpreted. That might be because humans have evolved to use mainly sight and touch for day to day activities (and so these inputs need careful attention), leaving our sense of smell as a more instinctive and subtle guide.
Many animals use smell as a means of communication too. The chemicals that animals secrete as messages are called pheromones. They can be warnings of danger, or even hints that the sender is on the lookout for a mate. There is little evidence to suggest that humans use pheromones but scientists think our ancestors may have done because there are parts of our nose which are no longer connected up to the brain. These may once have been part of the vomeronasal organ (the part of the nose that detects pheromones), but we have lost the use of it during evolution.
Did you know?
In the 1970s the US psychologist Martha McClintock observed that women appeared to change their menstrual cycles when exposed to the sweat of other menstruating females. She hypothesised that pheromones were responsible, and the research led to the urban myth that women’s cycles can synchronise. Recent analyses, however, indicate there were errors in McClintock’s data…
Find out more
Learn more about pheromones with this podcast from the University of Oxford.
Learn more about pheromones with this podcast from the University of Oxford (http://bit.ly/QWeXOQ).
Getting a whiff of the truth
But how do our noses detect chemicals anyway? Early in the 20th century scientists thought that olfactory receptors worked much like any other enzyme or protein: they have a well-defined three-dimensional structure and a specifically shaped cleft in the middle. Only molecules with a complimentary shape are able to fit inside the cleft, and so the idea became known as the ‘lock and key’ theory, because of the shape-dependant way the receptor and small molecule fit together.
You can imagine the nose like a dungeon with hundreds of locked doors leading to the brain (an analogy that works quite well, given the dark and slimy nasal environment). When a molecule wafts into the chamber it can only unlock a specific door, and the passage it activates tells the brain the identity of the intruder.
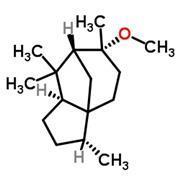
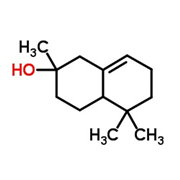
But alas, the concept of olfactory receptors being like locks has seemed over-simplified for decades. Molecules that have similar shapes would be expected to open the same locks, but as we’ve already seen (fig 1), structurally related molecules can smell utterly different. On the other hand, it’s not clear how cedramber and ambrinol (fig 2) could unlock the same smell receptors when they have such dissimilar structures; yet all three smell very alike.
Shaking possible answers out
Luca Turin, a Lebanon-born biophysicist fascinated with the mechanisms of smell, came up with an innovative theory to explain all this in 1996. It’s not just the molecule’s shape that’s important, according to Turin, but also how it vibrates once inside the receptor’s binding pocket. If it oscillates in just the right way it encourages an electron to ‘jump’ from one part of the receptor protein to another (in a process called quantum tunnelling), and this sets off a nerve impulse.
Turin’s theory can potentially explain the different scents of molecules with the same shape but different atoms. The vibrations of any given bond are decided not only by geometry, but also by the mass of the atoms at either end. Sulfur and oxygen are different sizes, so thiol and alcohol functional groups have different vibrational signatures.
Like all good theories, Turin’s made some predictions about smell that could be tested. One was that even subtle differences in atomic mass in similar molecules should result in odour variations. He stated, for example, that acetophenone and its deuterated analogue (acetophenone-d8) smell a little different (see ‘Subtle vibrations’).
Subtle vibrations
The mass of the two atoms at either end of a chemical bond helps determine the frequency at which it vibrates. It’s a little like a guitar string; if the atoms are heavier, they pull the ‘string’ taut and the vibration occurs at a higher pitch.
An easy way to change the mass of atoms without changing the shape of the parent molecule is to use isotopes: atoms which have the same number of protons but a different number of neutrons. Turin stated in the 1990s that acetophenone-d8, which has all its protons (1H) replaced with deuterium (2H), smells slightly different from the natural, all-hydrogen analogue.
In 2001 scientists from Canada set out to check if this ‘isotopes smell different’ rule would hold for a similar compound, benzaldehyde. They found that trained noses could distinguish regular and deuterated benzaldehyde quite easily. The two compounds smell similar (like ‘bitter almonds’), but not quite the same.
Interestingly, when they tried varying the isotope of carbon in the molecule (from regular 12C to 13C) people could detect a difference less often. That adds up, because the percentage mass difference between 12C and 13C is small, and would understandably affect the overall vibrations of the molecule less than the doubling of mass incurred when 1H is substituted with 2H. The results seemed to support Turin’s theory nicely.
But unfortunately this was not to be a case where the scientists all happily agreed with one another. In 2004 another team of scientists looked at Turin’s claim about acetophenone and acetophenone-d8, and disagreed with him: finding that, in general, their test subjects couldn’t distinguish the two molecules.
Adding up to a big stink

They also looked at another prediction of Turin’s theory – that molecules can add up to produce smells which none of the individual molecules give off in isolation. The classic example is a 1:1 mixture of benzaldehyde and guaiacol. Looking at these molecules, it is obvious that a mixture would contain the same functional groups as vanillin overall (aldehyde, methyl ether and phenol), but they wouldn’t add together to make something the same shape as vanillin (it would be nearly twice as big). Turin says that the 1:1 mixture does smell of vanilla though, adding that ‘the illusion is not perfect, but it is striking, because the vanilla character is absent from the components’.
The scientists then set out to test Turin’s reports themselves. They asked a group of test subjects to rate vials containing guaiacol, benzaldehyde, and a 1:1 mixture of the two on a scale from 1 (no vanilla character) to 13 (extremely vanilla). They tried the test at various concentrations, but at no point did the subjects find the 1:1 mixture more vanilla-like than either of the separate components. This apparently disproved another of Turin’s findings. The team ended their report of the study saying that ‘molecular vibrations alone cannot explain the perceived smell of an odorous chemical’.
To date, scientists still don’t agree on why similarly shaped molecules can smell so different, but we are certainly closer to the answer than we were before Turin’s hypothesis. His ideas may not be fully correct, but they have prompted people to ask some interesting questions that may eventually lead us to the answers.
Did you know?
Anosmia – the inability to smell – is more common than you might think with respect to specific chemicals. For example, asparagus contains methyl mercaptan, a thiol closely related to ethane dithiol (fig 1). Scientists think about 10% of people can’t smell this molecule, presumably because they are missing the requisite olfactory receptor.
Anosmia – the inability to smell – is more common than you might think with respect to specific chemicals. For example, asparagus contains methyl mercaptan, a thiol closely related to ethane dithiol (fig 1). Scientists think about 10% of people can’t smell this molecule, presumably because they are missing the requisite olfactory receptor. (For more information see http://bit.ly/VfDEHp).
Originally published in The Mole



No comments yet