Pottery vessels have been made for around 18,000 years. But how does clay extracted from the earth become a colourful pot, and what's the chemistry behind the process?
The process of firing a pot creates crosslinks between the hydroxyl groups in the clay
Oxides of the first row transition metals are the main sources of colour in pottery glazes
Origins

Making things from burnt clay has been part of human experience for many thousands of years. A small figurine of a woman is the earliest known object made of fired earth, dated to almost 30 000 years ago. The earliest known example of a pottery vessel was made around 18 000 years ago.1 Since then, the craft of pottery has developed in all parts of the world, both for the practical purposes of making usable vessels for food and storage, and as expressions of the instinct for art and ritual. About 7000 years ago the Egyptians discovered the art of glazing their pots. Subsequently the Chinese steadily improved kilns and so it was possible to produce more and more highly decorated stoneware and porcelain.
The immense strides in the making of pots were the result of patient trial and error by thousands of potters over thousands of years. A scientific approach to the process only became possible in the past two centuries, initially in establishing the compositions of the materials used. More recently, with the development of modern analytical techniques, the elucidation of their structures has been possible. Although much is now known about the materials and their structures, there are so many variables and the structures so complex, that the empirical approach still largely dominates pot making.
Making a pot
Forming a pot
There are many ways of forming a pot. Hand building is the earliest method and is still widely used. The potter's wheel was invented about 7000 years ago and has become the method of choice for many potters. In all methods it is essential for the clay to contain some water; not too much, because that makes the clay too soft and unworkable, or even turns it into 'slip' - a dispersion of clay in water. The essence of the plasticity is that the clay takes the shape given to it by applying a force and it keeps that shape unless some other force acts on it.
This property is readily understood if we consider that the layers are separated by a thin layer of water molecules which are linked to neighbouring layers via hydrogen bonds. These are weak but still significant forces. They are weak enough to allow the clay sheets to slide past each other when some force is applied, but strong enough to keep them in position once the force is removed. In other words, clay can be moulded into a shape, which it then keeps.
As the clay dries, water molecules escape from between the clay sheets, so these move closer together (the clay shrinks by 5% or more). The kaolinite hydroxyls become hydrogen bonded to the next layer, forming a stronger, firmer structure ('green ware'). If at this point the clay object is put into water it will disintegrate and can be returned into a workable state.
Clay
The feldspar group of minerals comprise around 60% of the earth's crust. They are aluminium silicates, also incorporating alkali and/or alkaline earth metals. A typical example is orthoclase, whose approximate composition is given as K2O.Al2O3.6SiO2. Over the geological timescale a great deal of feldspar has been eroded through a weathering process, mainly through the action of water. Although these rocks seem solid and eternal, over millions of years the effect of rain, made slightly acidic by dissolved CO2, does dissolve some of the alkali and alkaline earth metal oxides leaving the silicon and aluminium oxides:
K2O.Al2O3·6SiO2 + 2H2O → Al2O3.2SiO2.2H2O + K2O(SiO2) + 3SiO2
Feldspar a clay mineral in solution in the clay
The clay is either found where it had been formed or it can be carried by rivers and deposited elsewhere. When transported by water the particles continue to be ground finer and finer by the action of other rocks. They are also separated by size according to what settles out first. As a result, clay is a major component of soil all over the world, with a variety of properties according to the precise conditions that applied during its formation.2
Crystal structures
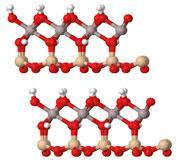
Crystallographic studies have established that the clay minerals are composed of sheets of tetrahedral silicon dioxide (SiO2) and octahedral aluminium oxide (Al2O3) linked through bridging oxygen atoms. On the aluminium oxide surface of the 'sheets' some of the oxygens are in the form of OH groups and there are OH groups within the structure as well. Broadly speaking, there are two main categories of clay minerals: those with one sheet each of the silicon and aluminium oxides and those with two sheets of silicon oxide enclosing a sheet of aluminium oxide.
The important mineral in pottery is kaolinite, which contains 1:1 silicon to aluminium oxides. The crystal structure shows plate-like particles, which are stacked in layers linked by hydrogen bonds. It is through the structure, properties and transformations of kaolinite that we can understand the physical changes involved in the making of a pot. Although there are deposits of virtually pure kaolinite, in practice it is always used as part of a mixture with other minerals, either because it is dug out of the ground in an impure state and used directly or because it is blended with other minerals (eg feldspar and quartz) to achieve the desired properties.2
Firing the pot
The dry pot is then heated to drive off some more water. Once its temperature reaches around 500ºC, the changes in it have become irreversible. At this point the clay is very fragile and crumbly, but it can no longer be reconstituted into the original workable state. This stage is described as the driving off of the so-called chemically bound water:
[clay]-OH + HO-[clay] → [clay]-O-[clay] + H2O(g)
The weak hydrogen bonds are replaced by stronger and shorter oxygen bridges (the clay may shrink a little further). When this happens the clay can no longer be recycled. Linking the neighbouring clay particles is a gradual process and if the firing is stopped at around 500ºC, enough of these crosslinks will be formed to prevent recycling, but not enough to strengthen the piece. At the same time the regular sheet-like crystal structure of kaolinite is being lost and amorphous metakaolinite is formed.3
Generally, the pot is first fired to about 1000°C to produce what is known as 'biscuit ware' with very slight further shrinkage. Biscuit ware is quite strong and porous; it readily absorbs water and dries again very easily. It is glazed by spreading a suspension of the glaze solids in water over the pot by pouring, dipping or spraying, and when it is dry, firing it again at the appropriate temperature for the clay and the glaze.
Stoneware vs earthenware
Pots can be classified according to the temperature they have been fired at - earthenware (1000-1150ºC), stoneware and porcelain (>1200ºC). In every case the clay composition has to be so that at the 'maturing temperature' it begins to vitrify and the partial melting of some of its components provides the 'glue' to provide its strength.
Other chemical changes take place during firing. These include burning off all organic matter often found in many clays, the decomposition of carbonates, which are common ingredients of many glazes, and further crosslinking of metakaolinite to give a three-dimensional network with the elimination of water. This process does not go to completion up to earthenware temperatures,4,5 but at stoneware temperatures all water is gone. It is difficult to believe that water is present in pots fired to earthenware temperatures, but easy to demonstrate:
Take two cups, one earthenware and one stoneware, and put water in both. Put them into a microwave and run it at full power for 2-3 minutes. The water in both should be hot; the handle of the earthenware cup will also be hot, while that of the stoneware cup will be cold. Since microwave ovens heat water by causing water molecules to move faster, the hot handle on the earthenware cup indicates the presence of free, mobile water molecules.
At stoneware temperatures, the metakaolinite undergoes transformation into mullite (3Al2 O3.2SiO2) which forms needle-like crystals, while the feldspar present melts into a glass, binding the mullite crystals together. These two structural changes account for the much greater hardness and strength of stoneware over earthenware.
Glazes
Most pots are glazed, ie they are covered by a thin coating of glass. This can be for aesthetic or for practical reasons, usually both. It is particularly important for pots holding food. The glaze usually has three main components:
- silicon dioxide to provide the main body
- aluminium oxide to enhance the viscosity of the glaze by crosslinking the silica networks
- fluxes, generally alkali or alkaline earth metal oxides, to lower the melting point of the mixture to the temperature of firing.2
In addition, it is common to include transition metal oxides to provide colour to the glaze.
A potter needs to consider three important properties of a glaze. These are the texture (rough or smooth), opacity (clear or opaque) and colour. The first two are best considered together in terms of the melting properties of solid mixtures.
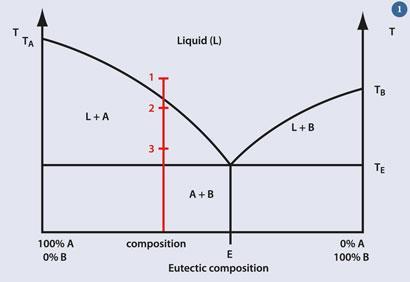
A generalised phase diagram (fig 1) illustrates the issues. Let us consider a mixture of composition indicated by the red line; when it reaches the temperature TE, it begins to melt. As the temperature rises the proportion of solid diminishes and the proportion of liquid increases until the last of the solid melts.
Point '1' corresponds to a glaze fully melted at the maturing temperature. Such a glaze is used when all the decoration has been done before glazing and if a smooth, shiny surface is required (fig 2).

Point '2' marks the maturing temperature when an opaque glaze is required; this should have a smooth surface (the glaze is mainly liquid), but it should include some solid to scatter the light and provide the opacity. In practice, the temperature in a kiln can vary considerably from the nominal value, which may complicate matters; fig 3 illustrates this well. The cup shown was made to have raised opaque spots on it, but it was placed too close to the heating elements, so the temperature range experienced by the cup straddled the liquid line. The spots on the right of the image were at '1' and those on the left were at '2', as intended.

The temperature indicated at point '3' produces a glaze that is still mainly solid with just a small portion melted. Such glazes feel rough to the touch, since the liquid is only enough to stick the solid components together and to the object; these are not recommended for use with food.
While the phase diagram illustrates the general phenomena, it represents a gross oversimplification of the real situation with glazes for two main reasons. One is that most glazes comprise more than two components, and the other is that the diagram presupposes that no chemical changes will take place to or between A and B. This is hardly ever the case, so any phase diagram representing the behaviour of a real glaze would be far more complicated.
Colour

The main minerals comprising the glazes are colourless. Both SiO2 (quartz) and Al2O3 (corundum) are known in nature in their pure states as white crystalline solids. They are also found in contaminated forms: amethyst and citrine are quartz contaminated with Fe, and ruby is corundum contaminated with Cr.When the contaminants in corundum are Fe, Co, Ti and V they are known as sapphires of various colours. In most glazes the colour is provided by oxides of the first row transition metals; in addition to those already mentioned, copper is also widely used. In pottery, the most common colouring oxides are those of iron, copper and cobalt. Of these, iron seems to be the most versatile; depending on the firing conditions and on what else is present in the glaze, it can give rise to red, yellow, brown, blue and green in various shades.
Unsurprisingly, the two dominant variables are the oxidation state and the environment of the transition metal ion. Potters using kilns heated by wood, gas or oil, have the option of using reducing conditions for part of the firing. The oxygen supply is restricted and the atmosphere in the kiln becomes rich in CO. If transition metals are present in low concentration in the glaze, they can be reduced to a lower oxidation state. The cup (fig 4) with iron glazes was fired under reducing conditions. The glaze on the inside contains 0.5% iron(III) oxide and the glaze on the outside 10%; the reducing power of the CO was enough to convert the iron in the inner glaze into Fe(II), but the concentration of iron in the outer glaze was just too high.

The vessel (fig 5) is glazed with a mixture containing CuO. Under oxidation conditions this would appear in the familiar blue or green of copper compounds. Under reducing conditions the copper is present as a mixture of Cu2O and finely dispersed elemental copper,6 hence the colour observed.
The fascination of pottery is that the variables are many, the possibilities are endless, and it offers opportunities for both artistic and scientific creativity. In addition, you can drink your tea out of your creations.
Stephen Breuer is a potter who previously taught chemistry at Lancaster University
References
- E Boaretto et al, P. Natl. Acad. Sci., 2009, 106, 9595 (DOI: 10.1073/pnas.0900539106)
- D Rhodes, Clay and glazes for the potter, 2nd edn, London, UK: A & C Black, 1973
- G Varga, Építoanyag[Building Materials], 2007, 59, 6
- S A T Redfern, Clay Miner., 1987, 22, 447 http://bit.ly/M3cstt (pdf)
- I Stubna, G Varga and A Trnik, Építoanyag[Building Materials], 2006, 58, 6
- A Paul, Chemistry of glasses, 2nd edn, p336-342, London, Chapman & Hall, 1990









No comments yet