Despite efforts to teach logic and critical thinking in the classroom, students will often give the answer that they think is expected. Perhaps a discredited theory from the 18th century can help students see how different conclusions can be drawn from the same experiment, suggests Mike Tingle

Science education should encourage students to make deductions and draw conclusions from experimental observations. However, at all levels, students often draw conclusions based on what they believe to be the expected ‘right answer’,1 rather than by applying logic to their own observations. To try and teach students to look more critically at experimental results in the classroom, and beyond, the old theory of phlogiston can prove useful once again.
My dictionary defines logic as the ‘science of reasoning, proof, thinking or inference; correct or incorrect use of argument.’ Good scientists apply logic to explain phenomena and develop theories, however, their inferences, arguments, and resulting conclusions, are not necessarily correct.
The phlogiston theory, for example, was accepted for more than 100 years. The theory held that materials that burned contained a fire-like element that was released as the object burned. So well entrenched was the theory, that when Joseph Priestley discovered oxygen in 1774 he called it ‘dephlogisticated air’, believing that the mercuric oxide (or calx) that he heated with sunlight had adsorbed phlogiston, removing it from the surrounding air.
Throughout history, theories that fit the known evidence have been accepted, holding sway until further evidence either disproves them, or gives rise to an improved version. In such a fashion, science and knowledge progress, but to students learning about this progression, their own modern knowledge can colour how they view superseded theories, and even their own science. The theory of phlogiston may seem nonsensical nowadays, but suspending disbelief and examining the evidence can help students to appreciate why it was accepted. It also provides a useful exercise in applying logic. Banishing all thoughts of oxygen requires students to explain experimental observations without being influenced by the modern ‘right answer’.
The phlogiston theory
In 1669, the German physician and alchemist Johann Becher published Physica Subterranea,2 describing the nature of minerals and other substances. He did not use the word ‘phlogiston’, but his ideas concerning combustion, metals and their extraction from rocks later formed the basis of the phlogiston theory.3
Becher reasoned that materials that burn must contain a combustible component, a ‘fire-element’, updating the alchemical principle that materials are composed of different proportions of four components, or elements: earth, air, fire and water. He dismissed fire and air, and instead proposed three forms of earth element: terra lapidea (stony / rocky earth), terra fluida (liquid earth) and terra pinguis (oily / fatty earth).
In Becher’s theory, terra pinguis is the supposed ‘fire-element’, the component that makes a material combustible. During combustion, this component is released into the air, with flames being the visible sign of its escape. The residue, eg wood ash, is ‘lighter’ (meaning less dense) than the original material – evidence that something has been lost, explained by the escape of terra pinguis. Similarly, heating a metal in air produces a calx, which is ‘lighter’ than the metal for the same reason.
In 1703, Georg Stahl, a German professor of medicine and chemistry, published an extended version of Becher’s theory,4 renaming ‘terra pinguis’ as ‘phlogiston’, from the Greek φλογισ (inflame). Stahl’s theory included the following ideas:
- All combustible substances contain phlogiston.
- The more phlogiston a substance contains, the better and more completely it burns.
- Combustion releases the phlogiston from the substance into the air. The flame indicates the rapid escape of phlogiston.
- Air is necessary for combustion because it absorbs the escaping phlogiston. Combustion in a closed container soon stops, because the air inside becomes saturated with phlogiston – it becomes phlogisticated air.
- Similarly, air is necessary for breathing. A creature placed in a closed container dies because the air cannot absorb any more phlogiston, so can no longer support life.
- The residue or ash left after combustion is called a calx.
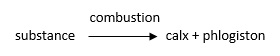
- Calcining (strongly heating) a metal in air also releases phlogiston, leaving the metal’s calx.

- This process is the same as combustion, but takes longer. The absence of a flame indicates that the phlogiston is escaping more slowly and gradually.
- The calx or ash is ‘lighter’ (less dense) than the metal because it has lost phlogiston.
Negative weight
But while the phlogiston theory explained many of the characteristics of combustion, there was a problem with calcining. Becher and Stahl knew that after heating a metal, although a calx may be ‘lighter’ with regards to density, its weight is actually heavier than the original metal. This had been shown by Jean Rey in 1630,5 and confirmed by Robert Boyle in 1673.6 To reconcile this fact with the phlogiston theory, phlogiston was attributed with negative weight – or ‘positive lightness’ – since it, or rather its flame, tends to rise, rather than be attracted downwards by gravity.

This conclusion was not as ridiculous as it now seems. In the classroom one can convincingly demonstrate apparent negative weight (mass) very easily with a simple experiment. A balloon filled with hydrogen gas, for example, will weigh less on a top-pan balance than an empty balloon.
You could challenge your students to explain this ‘impossible’ result. Even A-level students are usually puzzled by the result. They know that some gases are ‘lighter than air’, but the supposed phlogiston in this experiment appears to be lighter than no air at all. They realise this can’t be true, but few can explain the anomaly. With their minds focused on chemistry, rather than physics, they forget, and/or fail to appreciate, that buoyancy applies to objects floating in air, as well as in liquids.
At the time of Becher and Stahl, the experiment would have been performed using a pig’s bladder in place of the balloon, tying it to one pan of a beam balance.
Weighing ‘phlogiston’
- Place an empty balloon, about 10 cm of string and a few centimetres of sticky tape on a top-pan balance.
- Tare the balance to read zero.
- Fill the balloon with ‘phlogiston’ (aka hydrogen), tie the neck and tape it down onto the balance pan.
- The balance will now show a negative reading; ie the mass of phlogiston added to the balloon is negative.
Note: If there is no hydrogen cylinder available, shorten the string of a ‘phlogiston’ (aka helium) party balloon, tape it to the balance pan, and tare the balance. Burst the balloon and place it on the pan. The reading will be positive. Removing the phlogiston increases the mass, so phlogiston must have negative mass.
Explaining results without oxygen
In understanding why the phlogiston theory was used to explain experimental observations it is important to stress that, at the time, no-one knew about oxygen. To emphasise this, ask students to imagine travelling back in time to around 1770 – several years before the work of Priestley and Lavoisier led to the discovery of oxygen. Challenge them to explain the following experimental results, without invoking oxygen. (A practical approach for 11–14 year olds is available.)7
- Charcoal and coke burn leaving little ash (calx).
- Heating a metal calx with charcoal or coke produces the metal. (This is how copper, lead, tin and iron were obtained.) The metal weighs less than the calx, but is more dense.
- Similarly, heating the calx of copper, lead or mercury with inflammable air (a gas discovered in 1766) produces the metal.
- When calcined (heated in air), some metals form a calx more easily than others. (Though unknown until the early 1800s, the alkali and alkaline earth metals also fit the pattern. They are much less dense than other metals, and also burn to form a calx more easily.)
- The calx of an alkali or alkaline earth metal cannot be turned into the metal by heating with charcoal, coke or inflammable air.
- Heating a powdered metal with the calx of a different metal causes a reaction only if the first metal forms a calx more easily than the second; eg zinc reacts with lead calx, but lead will not react with iron calx.
- Adding iron, zinc or tin to an acid produces inflammable air and a solution of a metal salt. Reacting the acid with a metal’s calx instead of the metal gives a solution of the same salt, but no inflammable air.
- Inflammable air (hydrogen), burns leaving no ash. However it does produce a calx, which is gaseous, but condenses to a liquid and can be shown to be water. (Remember, you do not know about oxygen, so you do not know that water is hydrogen oxide.)
- Passing steam over red-hot coke produces two gases, one of which is inflammable air.
- Burning sulfur produces a gaseous calx, soluble in water (the calx of inflammable air) to form an acid. This acid reacts with many metals, producing inflammable air. The metals that react with the acid most easily and quickly are those that form a calx most easily when heated in air.
- Burning marsh gas produces water vapour (the calx of inflammable air). It also produces soot if the air supply is restricted, but not if there is plenty of air.

This exercise requires students to apply logic to the experimental results. Although they are not specifically told to invoke phlogiston instead of oxygen, most will probably do so. They will find that the theory, though false, does fit the evidence. From an 18th century perspective, after accepting negative weight as demonstrated above, the phlogiston theory is remarkably difficult to disprove.
...even modern scientific theories are just theories, not absolute fact
Having successfully explained a range of observations using a false former theory, remind your students that even modern scientific theories are just theories, not absolute fact. Hopefully, they will be encouraged to adopt a more inquiring mind, rather than blindly accepting received wisdom.
Eventually, the dominant theory of phlogiston was disproved. Antoine-Laurent Lavoisier showed that combustion requires a gas that has weight (oxygen) and could be measured by means of weighing closed vessels. By using closed vessels Lavoisier negated the buoyancy that had disguised the weight of gases of combustion, solving the weight paradox and setting the stage for a new theory of combustion. Students often adjust or misinterpret their experimental results to fit the ‘right answer’. In that mode they will never follow in the footsteps of Lavoisier, Hennig Brand, William Perkin, Lord Rayleigh, or Alexander Fleming, or many of the other scientists who made important discoveries by following up unexpected results.
Mike Tingle is a freelance author and educational consultant specialising in applied science
References
- M Allen and E Briten, School Science Review, 2012, 94(346), 112
- http://gallica.bnf.fr/ark:/12148/bpt6k84226t
- https://en.wikipedia.org/wiki/Phlogiston_theory
- http://www.sethi.org/phlogiston/data/timeline.html
- https://en.wikipedia.org/wiki/Jean_Rey_(physician)
- http://encyclopedia2.thefreedictionary.com/Robert+Boyle
- D Warren, Chemists in a social and historical context, p15. Royal Society of Chemistry, 2001









No comments yet