Rather than anticipating mistakes in practical work, let students make them – then use it as a learning point
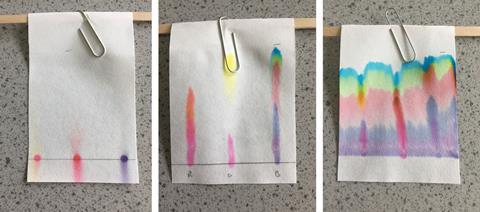
When students are about to carry out the paper chromatography of ink, most science teachers say: ‘make sure you draw the start line in pencil.’ It’s a common reminder, but maybe we should be saying: ‘make sure you try drawing the start line in ink.’
At a recent session on assessment objective 3 (AO3) at GCSE level, someone suggested getting the pupils to make the mistakes we talk about (where safety allows). That’s why I’m suggesting letting pupils draw the start line in ink.

I’d done this as a demonstration before but I hadn’t given it as much thought as I should. For this particular experiment, let pupils make common mistakes. Then discuss why each mistake causes a problem and how to fix it.
| Mistake | Problem caused | Solution |
|---|---|---|
| Incorrect solvent used | Ink insoluble so chromatography doesn’t occur. | Have pupils use a different solvent, eg ethanol. |
| Start line drawn in ink | Ink dissolves/runs in solvent. | Use pencil as pencil is insoluble in water. |
| Spots under the solvent | Ink will dissolve in the solvent and wash off the paper. | Ensure the solvent is below the start line. |
The Education Endowment Foundation’s Improving secondary science report supports this, recommending that you seize the opportunity when experiments go wrong, to ‘use scientific reasoning to explain the unexpected’. The idea of modelling mistakes and talking through your thought process also links nicely to Barak Rosenshine’s Principles of instruction paper. How thinking aloud builds student understanding can help with this.
Sometimes I’ve been guilty of expecting pupils to be able to automatically suggest sensible improvements to experiments that have gone wrong. Personally, I have found it better for pupils to see mistakes in action (again where safety allows). Consequently pupils have answered AO3 questions better, more were able to articulate what problem the mistake would cause and suggest a credible improvement.
If it is unsafe to let a pupil make a particular mistake, eg adding anti-bumping granules to a liquid near its boiling point or having a completely sealed distillation set-up, use diagrams of incorrect set-ups and discuss what would be needed to fix that particular mistake.
For the required practicals listed by the common exam boards remember it is the skills and equipment being tested. It may not be the chromatography of ink examined, it could be plant pigments, indicators, amino acids etc.
Using alternative methods certainly helps, particularly if you follow a spiral curriculum. For example, at KS3, when teaching chromatography, students could perform paper chromatography of ink and then at KS4 they can do chromatography of pH indicators/amino acids/plant pigments and calculate Rf values. But again remember, students could be asked to identify mistakes made in that experiment.
| GCSE required practical (AQA) | Example mistake(s) |
|---|---|
| Making salts |
Incorrect metal oxide/acid used leading to the formation of an incorrect product. Not adding the metal oxide in excess. |
| Temperature changes |
Not using a polystyrene cup/lid. Bulb of thermometer not submerged fully in the reaction mixture. |
| Rates of reaction |
Bung not added quickly. A different person each time looks for when the cross disappears during the ‘disappearing cross’ experiment. |
| Water purification |
Having a completely sealed system. Not adding anti-bumping granules. Water in and out incorrect, so cooling inefficient. |
| Electrolysis |
Electrodes touching. |
| Neutralisation (titration) |
Unsuitable indicator used. White tile not used. |
| Identifying ions |
Hydrochloric acid used instead of nitric acid during the halide test. |
Check out the relevant GCSE chemistry practicals for all English and Welsh exam boards.
AO3 questions will always challenge pupils. In my experience, they struggle with them. However, AO3 makes up approximately 20% of the GCSE chemistry exam papers and for students to really access the top grades they need to have a sound understanding of error and be able to offer improvements.
So, the next time you carry out the paper chromatography of ink experiment, try letting your students draw the start line in ink.














2 readers' comments