An effective visual aid you can count on in your electrochemistry lessons
Mention chemical cells in a department meeting and, chances are, you’ll be met with a groan. A topic synonymous with misconceptions and confusion at 14–16, and calculation-dense at 16–18, it is one I dread to see on my long-term plans. I’ve never found a satisfactory way of explaining it which is both accessible yet distinct enough to avoid causing confusion with electrolysis. Discussions in my department led to a colleague demonstrating how to analyse experiments using a number grid and I was left gobsmacked at its simplicity. I decided to use this as the basis for my explanations and calculation work, and haven’t looked back since.
What prior knowledge is needed?
At 14–16:
- Knowledge of ions as atoms which have lost or gained electrons
- Reactivity series of metals
- Metal displacement reactions
- Oxidation and reduction in terms of loss and gain of electrons
- Half-equations
At 16–18:
- The standard hydrogen electrode
- Standard electrode potentials
- Some knowledge of IUPAC convention for notation of electrochemical cells
How to set up a number grid
I start by encouraging students to consider a reaction they already know: a zinc–copper cell. Most students will know from the reactivity series that zinc is more reactive. This can be confirmed by showing the half-cell equations and the electrode potentials.
Zn2+ (aq) + 2e- ⇌ Zn (s) Ecell = -0.76 V
Cu2+ (aq) + 2e- ⇌ Cu (s) Ecell = 0.34 V
I then give them a step-by-step method to lay out their number grid:
- Place the more reactive, negative cell on the left and the more positive (less reactive) on the right.
- Add the standard hydrogen electrode at 0.00 V – for GCSE and equivalent courses, just put 0.00 V.
- Add the electrode potentials to the grid (A-level and equivalent courses only).
- Electrons should flow from left to right, so label the left half-cell as oxidation (supplying electrons) and the right half-cell as reduction (accepting electrons).
- Identify the anode (where oxidation occurs) and the cathode (where reduction occurs).
- Calculate the electromotive force (EMF), Eocell = Ereduction - Eoxidation
NB: students may spot that the gap between the two cells, and therefore the EMF, should be 1.10 V without the need for the last step.
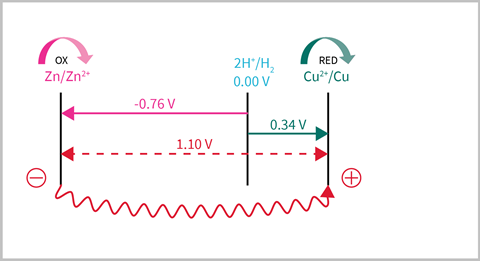
Using this method also provides a nice stepping stone for writing the standard convention of the cell using IUPAC notation:
Zn (s) | Zn2+ (aq) || Cu2+ (aq) | Cu (s)
A non-metal example:
My A-level students often become confused when applying this to non-metals, as they tend to forget the basic principles of electron transfer. I encourage them to look at the electrode potentials and map it out.
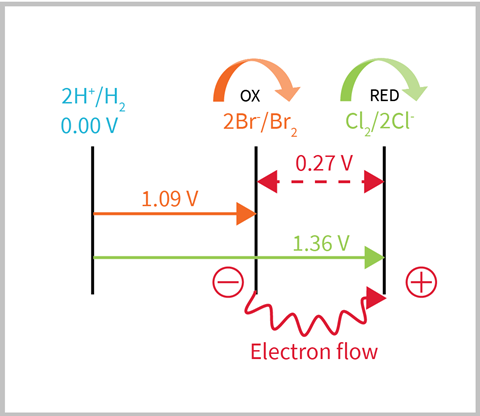
This method is also very useful for explaining why the reverse reaction doesn’t happen; either because the electrons can’t flow in the opposite direction, or you could calculate the EMF to show that the reaction is not feasible.
Eocell = 1.09 - 1.36 = -0.27 V
Predicting cell potentials
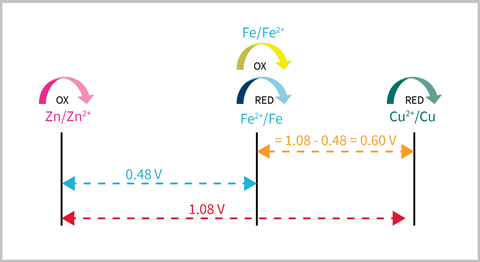
Number grids really come into their own when used to predict unknowns with given potentials. Students can use a simple practical to measure electrode potentials for a zinc–iron(II) and a zinc–copper cell to produce a number grid of their results. They can then use this to predict the cell potential for an iron(II)–copper cell and test their ideas by setting up the cell.
Misconceptions and how to tackle them
Depending on your teaching order, there may be some misconceptions from other topics. For example, students are taught the anode is positive and the cathode is negative in electrolysis. Using numer grids, I highlight this difference by asking students the charge of an electron (negative), and so which electrode it would flow towards (positive). By reinforcing that oxidation always occurs at the anode, students can then map out the direction of electron flow on their grid.
Students sometimes think there should be an external voltage applied to electrochemical cells due to confusion with electrolysis and circuits in physics. The main visual aid students are given when studying electrochemical cells is cell diagrams which contain a voltmeter – a piece of equipment students usually encounter in physics when measuring potential difference. By removing the whole concept from these physics-style cell diagrams and showing electrode potentials on number grids, you can overcome any potential sources of cross-curricular confusion.
Number grids are now a firm favourite for me when teaching electrochemical cells. They have been especially helpful as an initial visual aid to build understanding and confidence in a topic which can feel unfamiliar. Taking an abstract idea and mapping it out in an accessible way helps develop a clear method for solving problems. This allows for an easier transition into tackling more complex examples; both for your students and you as their teacher.
More resources
- Investigate electrochemical cells with two microscale experiments using this video and additional resources with your 16–18 learners.
- Use this Starter for ten which includes questions on redox reactions, standard electrode potentials, calculations involving electrochemical cells and their appplications.
- Give your 14–16 learners context when studying electrochemical cells with this one-slide summary and questions on more sustainable batteries.
- Highlight the applications of electrochemistry and link to careers, such as this job profile of a PhD researcher studying the best electrode materials for lithium- and sodium-ion batteries.
Louise Hussein













1 Reader's comment