Tips to help students grasp organic mechanisms
Organic chemistry can come as a bit of a shock to older students. Up to the age of 16, they have been used to just learning the content – the structures of particular functional groups, alkane chains and a few reaction types. The simplistic nature of many associated questions meant these could be regurgitated in exams. So organic chemistry can be perceived as quite easy and a bit of a memory test. Then, suddenly, they are faced with organic reaction mechanisms and everything becomes a lot more complicated.
Laying the foundations
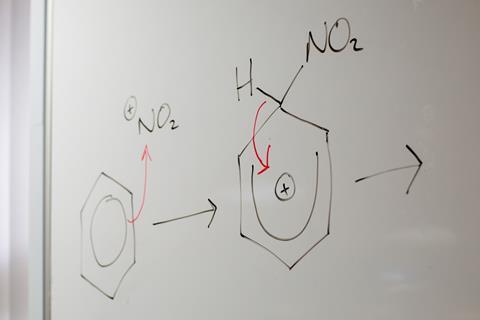
It’s tempting for students to approach mechanisms as just another thing to commit to memory, but ultimately this leads to superficial learning and insecure understanding. A curly arrow represents the movement of a pair of electrons and ideally the teaching of mechanisms should be approached from first principles. This needs students to be confident identifying regions of high and low electron density and so a recap of electronegativity might be needed. It may feel like progress is being slowed by recapping these basic underlying principles, but it will pay off in the long term.
Strategies that support retention of understanding
Develop confidence with whiteboards and colour
Monochrome mechanism outlines are not very memorable. Encourage students to use different colours for the structures and the arrows. Lower achieving students may be reluctant to commit pen to paper for fear of making a mistake. Whiteboards and coloured markers can be useful in building their confidence, allowing them to make errors and easily correct them. They can then copy out their correct answers neatly onto their notes. Don’t be afraid to invite pupils up to the whiteboard to add arrows to schemes and articulate their thinking.
Dual coding mechanisms
Mechanism outlines are essentially pictures. Dual coding is a study method that has significant evidence of efficacy. In dual coding, words are combined with pictures in order to make a stronger memory. To dual code mechanisms, combine sentences that justify each arrow with the outline. It is useful to first model this process with a visualiser or on the whiteboard, to show the level of detail required.
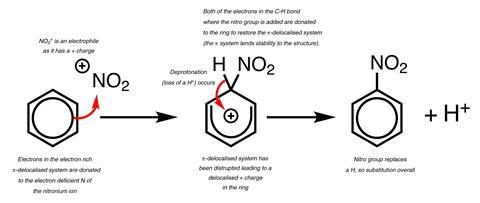
Error spotting
Students may find it difficult to spot errors in their own mechanisms, in much the same way as it is often difficult to spot your own spelling mistakes. Peer marking of class exercises can be a good way to expose students to different ways of drawing mechanisms, both correct and incorrect. Where class sizes are small or cohorts are homogeneous, then class or homework exercises to spot errors can provide useful practice. Examiners reports often discuss significant errors noted for particular questions each year so can provide a basis for modelling common errors. Handwritten mechanisms tend to have greater impact for students than those prepared using computer software. Model neat and tidy outlines in your own hand but remember to scan and save them so you don’t have to remake your examples each year.
Practice makes permanent
Like with learning any new concept, practice is key. The design of the practice is as important as the quantity. There are many different aspects to students being successful in questions about organic mechanisms so try not to teach them too quickly or to start mixing the different mechanism types together too early. Provide enough practice of the different aspects of individual mechanisms each time a new mechanism is taught. Then begin to bring different mechanisms together in the same exercise so students can practise discriminating between them.
Aspects of success in mechanism questions
- Identifying the reagent(s) and reactive species in a given transformation.
- Identifying the functional groups present in the starting materials.
- Drawing the reacting molecules, eg starting materials and products from their IUPAC names.
- Identifying the type of mechanism occurring for a given transformation.
- Identifying regions of high and low electron density and accurately labelling dipoles.
- Identifying and labelling the charges on atoms that have fewer or more bonds than usual as a result of electron movements.
- Outlining mechanisms on skeletal formulas (where required).









8 readers' comments