A versatile compound used in cancer treatment, solar cells and even making beer
Caesium is one of those elements that feels unfamiliar, which leaves a compound like caesium chloride inevitably obscure. Yet, while only about 20 tonnes of this colourless crystalline substance are produced each year, it has a surprisingly wide range of applications.
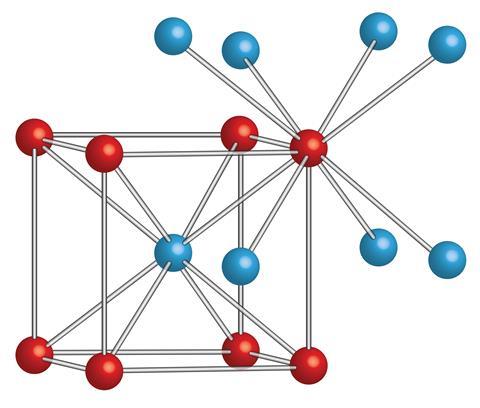
Structurally, the salt is a simple chloride like the more familiar sodium chloride, but because caesium is a lot closer to chlorine in size, it forms a crystalline structure where each caesium ion is surrounded by eight chlorine ions at the corners of a cube. By contrast, in sodium chloride, each ion is surrounded by six of its counterpart in an octahedral shape.
The best-known uses are probably those involving a radioactive form of the salt. Caesium chloride with non-radioactive caesium-133 occurs naturally, as a trace constituent in some minerals and in mineral water (which is where caesium was discovered). It is most concentrated in a mineral called pollucite, also containing aluminium and silicon among other constituents, where caesium makes up around 20% of the whole. Elemental caesium itself is usually produced from the caesium chloride extracted from the mineral. But to create the radioactive form of the compound, it is enriched with caesium isotopes, particularly caesium-137, produced in nuclear reactor waste.
This radioactive form is used to treat cancers and, unlike most other medical radioisotopes, it is water soluble. Caesium chloride packs a lot of radioactivity into a small volume, making it ideal for treatments where the radioactive material needs to be accurately sited.
A dangerous glow
Generally, the radioactive form is kept in highly secure containers, but in 1987 a caesium chloride source containing about 93 g of the salt was stolen from a disused hospital in Goiania, Brazil. The thieves partially broke open the container and sold it on for scrap. Because the exposed caesium chloride gave off an eerie blue glow, it became a popular talking point. Samples of the glowing powder were shared around. Even the scrapyard owner’s six-year-old niece used it as a skin decoration. She was one of four who died as a result of the exposure, with over 200 more suffering significant radioactive contamination.

When a compound like caesium chloride gets a bad name, it’s hard to see it as anything other than a problem. But it has a beneficial medical use, and many of its applications involve the harmless stable form.
In fact, the non-radioactive form has some interesting tricks up its sleeve. One of the most common uses of the compound is in ultracentrifugation. Here, extremely high speed centrifuges, producing a g-force of up to a million times that of Earth surface gravity, are used to separate out materials using a gradient of increasingly high concentrations of a dissolved compound. Caesium chloride has proved ideal as the dissolved compound for splitting off nucleic acids, because of its good water solubility and relatively high density. A sample of DNA can be broken down this way into sections. Similarly, mitochondrial and nuclear DNA can be separated, or the simpler RNA molecule can be removed from DNA samples.
Although caesium chloride itself is only slightly electrically conductive, a thin layer of it can enable the production of electrically conductive glass, and can boost the efficiency of solar cells.
It is also found in specialist ultraviolet excimer lamps, solders and, perhaps most surprisingly, in the production of beers and mineral waters. Apparently it improves the taste of mineral water, though it’s hard to see exactly how – one commentator described sampling caesium chloride: ‘[It] does not taste very good at all, and sort of burns a bit. It’s very metallic in taste and has a horrible aftertaste.’









1 Reader's comment